Abstract
Background: Older people have the highest incidence of melanoma and the population in most Western countries is ageing. We evaluated how the gap in incidence and survival between younger and older patients has developed during the past decades.
Material and methods: All patients diagnosed with cutaneous melanoma between 1989 and 2015 (n = 84,827) were identified from the Netherlands Cancer Registry. Elderly were defined as aged ≥70 years. Differences in patient and tumor characteristics were described, age-specific incidence rates were calculated, and relative survival (RS) and multivariable analyses estimating the Relative Excess Rate of dying (RER) were conducted
Results: In older men, the melanoma age-standardized incidence increased from 18 to 103/100,000 person-years (py) between 1989 and 2015 and in older women from 23 to 70/100,000 py. In younger men and women, it increased from 8 to 21 and from 13 to 28/100,000 py, respectively. Median Breslow thickness declined from 1.8 to 1.1 mm and from 1.6 to 1.1 mm in older men and women (2003 versus 2015), and from 1.1 to 0.9 mm and 0.9 to 0.8 mm in younger men and women. In older men, 5-year RS increased from 67% (95% CI: 63%–72%) in 1989–1997 to 85% (95% CI: 83%–87%) in 2007–2015 and in older women from 81% (95% CI: 78%–85%) to 89% (95% CI: 87%–91%). In younger men and women, RS increased from 82% (95% CI: 81%–83%) to 90% (95% CI: 90%–91%) and from 92% (95% CI: 92%–93%) to 96% (95% CI: 95%–96%). After case-mix correction , older men and women no longer showed an improved survival over time (RER 2010–2015 versus 2003–2009: 0.97; 95% CI: 0.81–1.16 and 0.95; 95% CI: 0.79–1.16). Whereas in younger men and women survival remained improved (RER 0.75; 95% CI: 0.67–0.83 and 0.77; 95%CI: 0.67–0.89).
Conclusion: The gap in melanoma incidence between younger and older people is increasing due to a strong increase in incidence in older adults. Disparities in survival are declining, related to a narrowing gap in Breslow thickness.
Introduction
While cutaneous melanoma is one of the cancer sites with a rather high risk among younger persons, the risk of being diagnosed with a melanoma is still considerably higher among older persons.
The Netherlands currently ranks third in terms of melanoma incidence in Europe, and its incidence is still increasing [Citation1,Citation2]. Like many other Western countries, the population is ageing. During the next decade, the number of elderly (aged ≥65 years) is expected to rise with 30% [Citation3]. Due to the ageing population and the higher risk of melanoma seen in older people, a fast-growing group of older melanoma patients will be encountered in clinical practice in the near future.
Being diagnosed at an older age is generally a poor prognostic factor in cancer. The worse prognosis of older patients is related to a late diagnosis, presence of more poor histological features, less treatments offered or used by patients and a higher number of comorbidities or lower performance score compared to younger patients [Citation4–9].
Despite the growing number of older patients, most clinical trials are still focused on the younger and more fit patients. If older patients are included, they are usually not representative for the entire cohort of older cancer patients. Consequently, the knowledge concerning the safety and efficacy of treatment regimes in older patients is often limited.
With this nationwide study, we aim to provide insight into the burden of melanoma in elderly patients. Besides, we will evaluate how the gap in incidence and survival has developed during the last three decades and relate this to changes in patient and tumor characteristics.
Material and methods
Patient selection
Patients were identified from the Netherlands Cancer Registry (NCR). The NCR is a population-based registry based on notification by the automated nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA), and complemented by other sources such as national registry of hospital discharge and radiotherapy institutes. This comprehensive registry covers the entire Dutch population.
After notification, fully trained registry clerks routinely extract data on patient and tumor characteristics and treatment from pathology reports and patients’ files in all Dutch hospitals. Information on vital status (alive, emigrated or dead) and date of death or emigration is annually retrieved from the database of deceased persons of the Central Bureau of Genealogy and the municipal demography registries (GBA).
Topography and Morphology are coded according to the International Classification of Diseased for Oncology [Citation10,Citation11] and tumor stage according to Tumor-Node-Metastasis (TNM) classification (International Union Against Cancer, 6th (2003–2009) and 7th (2010–2015) edition) [Citation12,Citation13]. The pathological T, N and M stage was used and supplemented by information of the clinical stage in case pathological stage was not available. For overall TNM stage, we assumed that if no information on lymph node or distant metastases status was recorded in a patient’s medical file they were negative.
Comorbidities were routinely collected for patients diagnosed the southern part of the Netherlands and recorded according to a slightly adapted version of the Charlson classification [Citation14,Citation15].
For this study, all consecutive patients diagnosed with a primary invasive cutaneous melanoma in the Netherlands between 1989 and 2015 were selected from the NCR. Patients diagnosed at autopsy were excluded (n = 168). Variables that were selected from the NCR included age at diagnosis, histology (nodular melanoma (NM)/superficial spreading melanoma (SSM)/lentigo maligna melanoma (LMM) and other/unspecified), Breslow thickness, TNM stage, lymph node status (positive/negative/unknown), number of comorbidities and vital status. We chose to define older patients as aged ≥70 years at time of diagnosis because age-specific incidence rates peaked at the age of 70 [Citation1].
Statistical analyses
Trends in melanoma incidence were analyzed by calculating annual age-standardized incidence rates by age category (<70 years (younger) and ≥70 years (older)), and age-specific incidence rates (5-year age categories) by three equal time periods (1989–1997, 1998–2006, 2007–2015). For age standardization, we used the 2000 Dutch population (stratified by <70 and ≥70 years) as standard. All analyses were stratified by sex. Changes in incidence were evaluated by calculating the estimated annual percentage changes (EAPCs) and their corresponding 95% confidence intervals (CI). To calculate this, a regression line was fitted to the natural logarithm of the rates, using the calendar year as regressor variable [i.e., y = ax + b where y = ln(rate) and x = calendar year, then EAPC = 100 × (ea−1)].
Differences in tumor and patient characteristics between age groups (<70, 70–79, 80–89 and ≥90 years) and between time periods (2003–2009 versus 2010–2015) in older patients were evaluated using chi-square tests and Wilcoxon Rank sum tests. These analyses were performed from 2003 onwards because of changes in TNM classification and the availability of Breslow thickness in the NCR. Subgroup analyses including comorbidities were performed since comorbidities were only routinely collected in the South Eastern region of the Netherlands (n = 6980, 12% of the study population).
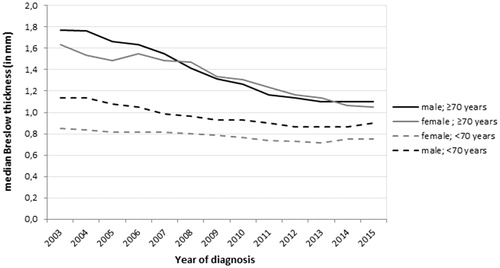
A graph of the median Breslow thickness per year and age category was constructed to show the trends over time. Median Breslow thickness was smoothed by calculating the three-year moving averages in order to demonstrate time trends more clearly.
To obtain an estimation of disease-specific survival, we calculated one- to five-year relative survival (RS) according to the Ederer II method [Citation16]. In the RS analyses, the ratio of observed to the expected survival based on a sex, age and calendar year matched population was calculated. Survival time was defined as date of diagnosis to date of death, emigration or until 1 January 2018, whichever came first.
Analyses were stratified by period of diagnosis and sex, or by stage at diagnosis.
To identify the effect of age on excess mortality (relative excess risk of dying (RER)) due to melanomas and to identify factors associated with excess mortality in younger and older patients, we performed multivariable regression models using the Poisson generalized linear model framework. In these models, we included the log-transformed Breslow thickness.
All analyses were performed using STATA/SE 14.1 (StataCorp, College Station, TX, USA). A two-sided p value of <.05 was considered statistically significant.
Results
A total of 84,827 patients with invasive cutaneous melanoma diagnosed between 1989 and 2015 were identified from the NCR, 19,507 (23%) of them were aged ≥70 years at time of diagnosis. The proportion of older patients increased from 17% in 1989 to 29% in 2015. Median age at diagnosis increased with 11 years (from 50 years to 61 years) during the same calendar period.
Trends in incidence
shows age-standardized incidence rates/100,000 person-years (py) of melanoma by sex and age category (<70 years and ≥70 years) between 1989 and 2015.
Figure 1. Age-standardized incidence rates of cutaneous melanoma, by age category and sex (1989–2015).
Rates are age-standardized using the 2000 Dutch Population. CI: confidence interval; EAPC: estimated annual percentage change.
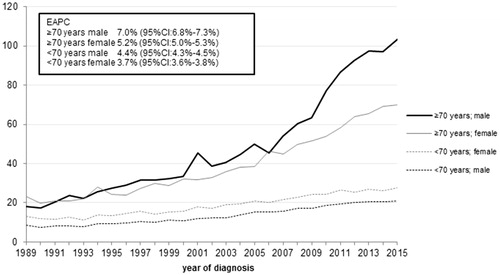
An increase in incidence was seen among all groups. In younger men and women (<70 yr), age-standardized incidence increased steadily, whereas older men showed the steepest increase with an EAPC of 7.0% (95% CI: 6.8%–7.3%). In 2015, their incidence rate was nearly 6 times higher compared to 1989. In older women (≥70 yr), incidence increased threefold during the same time period with an EAPC of 5.2% (95% CI: 5.0%–5.3%).
Incidence rates, by 5-year age categories and period of diagnosis, showed an increasing incidence in nearly all age categories (in both men and women). The incidence increased most between the two most recent time periods (2007–2015 versus 1998–2006) (Supplementary Figure 1). In 2007–2015, age-specific incidence rates in women stabilized after the age of 65 years with an incidence rate around 60/100,000 py. In men, age-specific incidence rates continued to increase with advancing age and peaked at 97/100,000 py in men aged ≥85 years.
Patient- and tumor characteristics by age category
With advancing age, the proportion of patients diagnosed with thicker melanomas (Breslow thickness >2 mm) increased (). Also more older patients had an unknown Breslow thickness. SSM was the most frequently diagnosed histological subtype among all age categories in both men and women, but the proportion decreased with advancing age. NM and LMM were more frequently seen with increasing age in both sexes.
Table 1. General characteristics of patients diagnosed with cutaneous melanoma in the Netherlands, by age category and sex (2003–2015).
In younger male patients, most melanomas (54%) were seen at the trunk, and 12% was located at the head or neck. Contrary, in the oldest male patients (≥90 yr) most melanomas (58%) were seen at the head and neck, and only 24% was diagnosed at the trunk. The most common site for melanomas in younger females was the lower limbs (38%), while 7% was located at the head or neck. In the oldest age category (≥90 yr), the predominant location was the head and neck (39%) and fewer melanomas were found at the lower limbs (27%).
Time trends of patient- and tumor characteristics of older melanoma patients
In both older men and women, period of diagnosis was statistically significantly associated with Breslow thickness, tumor localization, histological subtype and lymph node status (). In the more recent years (2010–2015 versus 2003–2009), older men and women were more often diagnosed with thin (<1 mm) melanomas, and with truncal melanomas. The proportion of SSM increased from 48 to 69% in older men, and from 47 to 57% in older women. Lymph node status was more often known over time, with more patients having a negative lymph node status (63% in 2003–2009 versus 71% in 2010–2015 for both men and women). Whereas period of diagnosis was statistically significantly associated with distant metastases (p = .03) in older women, we did not find an association in older men (p = .3).
Table 2. Patient and tumor characteristics of older cutaneous melanoma patients (aged ≥ 70 years), by the period of diagnosis.
Median Breslow thickness declined strongly in older patients (). In 2003, the median Breslow thickness of older men was 1.8 mm (Interquartile range (IQR): 0.9–4.0 mm) versus 1.1 mm (IQR: 0.6–2.6 mm) in 2015. In older women, the median Breslow thickness decreased from 1.6 mm (IQR: 0.7–3.8 mm)) to 1.1 mm (IQR: 0.5–2.5 mm). During the same time period, Breslow thickness decreased with 0.2 mm in younger men (from 1.1 (IQR: 0.7–1.3 mm) to 0.9 mm (IQR: 0.5–1.7 mm)) and 0.1 mm in younger women (from 0.9 mm (IQR: 0.6–1.5 mm) to 0.8 mm (IQR: 0.5–1.3 mm)).
Survival
Older patients showed worse RS compared to younger patients (Supplementary Figure 2). Over time, survival improved in all patients. The improvements were largest in older men, but also started at a lower level. Their 5-year RS increased from 67% (95% CI: 63%–72%) in 1989–1997 to 85% (95% CI: 83%–87%) in 2007–2015. In older women, 5-year RS increased from 81% (95% CI: 78%–85%) to 89% (95% CI: 87%–91%) between the same time periods. Also in younger patients survival improved, but differences in survival between younger and older patients declined over time. In men, the gap declined from a 15 percentage point (pp) difference in 1989–1997 to a 5 pp difference in 2007–2015, and in women from a 11 to a 7 pp difference, respectively.
Compared to younger patients, RS by TNM stage revealed poorer outcomes of older patients diagnosed with a stage II, III or unknown stage melanoma, but not for patients with a stage I or IV melanoma (2003–2015) (Supplementary Figure 3). Survival of stage I melanoma slightly exceeded the survival of the general population in older patients, whereas it approached the general population’s survival in younger patients.
In multivariable survival analysis (adjusted for sex, histological subtype, localization, Breslow thickness, lymph node status and distant metastases), age was identified as an adverse prognostic factor (RER ≥80 years versus <70 years: 2.21; 95% CI: 1.92–2.55) (data not shown). Analyses stratified by age category showed a statistically significantly lower RER of dying in the most recent time period in younger patients. In older patients also a lower RER was seen in the univariable analyses, however, this association disappeared after adjustment for other prognostic factors ().
Table 3. Relative excess rate of dying due to melanoma, by age category and sex (2003–2015).
Analyses were repeated with only period of diagnosis and Breslow thickness entered into the models. Also in these models, period of diagnosis was no longer associated with excess mortality (2003–2009 versus 2010–2015) in both older men (RER 0.97; 95% CI: 0.81–1.16) and older women (RER 0.99; 95% CI: 0.83–1.12) (data not shown).
For subgroup analyses, we added comorbidity to the models, this resulted in similar associations between the prognostic factors and survival as observed in the models without co-morbidity (data not shown).
Discussion
Incidence of melanoma is increasing in all age categories. However, a disproportional increase is seen among the elderly, particularly among older men. They have shown a nearly 6-fold increase in incidence since 1989. This unfavorable trend among older men is in accordance with observations from other countries [Citation17–19].
A possible explanation for the disproportional increase in incidence among older people (men) is that they gained less advantage of public health campaigns compared to younger people. A recent study in Australia showed stabilizing and falling trends in incidence among young people, but not yet in the elderly (aged > 60 years). A comparison of birth cohorts revealed that the largest decline in age-specific melanoma incidence rates was seen in the cohorts that were raised after the introduction of public health campaigns and thus grew up with greater awareness of melanoma risk compared to earlier birth cohorts [Citation19]. An explanation for the higher susceptibility to melanoma of particularly older men might be a higher lifetime UV exposure (more total time spent outdoors), combined with poorer sun protection behaviours [Citation20,Citation21].
In the Netherlands, public health campaigns aimed at increasing awareness of skin cancer started in the late 1980s. While it may be too early to see a levelling-off or decrease in incidence in the older age categories yet, it may have resulted in an increased awareness of the signs and symptoms of melanoma, and subsequently an earlier detection.
Older patients are still diagnosed with thicker melanomas. However, median Breslow thickness has decreased considerably during the recent years, particularly among older patients. This may suggest that, regarding melanoma awareness, older people (particularly men) are catching up with younger people. A previous study concluded that it seems likely that overdiagnosis of thin lesions contributed to the increasing trends in melanoma incidence rates in the Netherlands [Citation22]. This may also partly be the case for the older patients in our study. Due to more awareness, possibly more (thin) melanomas were detected and treated, which would not have resulted in morbidity and mortality otherwise. However, analyses stratified by Breslow thickness showed a rising incidence of all (thin (<2mm), intermediate-thickness (2–4mm), and thick (≥4mm)) melanomas in older patients during 2003–2015 (data not shown). This suggests that the increasing melanoma incidence among older people is not entirely due to overdiagnosis, even though the results confirmed that incidence of thin melanomas increased most rapidly.
The gap in median Breslow thickness between the younger and older patients declined, particularly because of the decreasing Breslow thickness among older patients. This observation is in contrast with an earlier study among Dutch melanoma patients, reporting a less prominent decline in Breslow thickness among older patients than among younger patients [Citation23]. Nonetheless, in contrast with this previous study, we used a continuous measure of Breslow thickness (median), instead of a categorical variable, and included a more recent time period (1994–2008 versus 2003–2015), which may explain the different results.
Besides a declining gap in Breslow thickness between younger and older patients, also disparities in RS declined over time. This seems related. Both in younger and older patients, we observed an increase in survival. But also here, the largest improvements are seen in the elderly. Survival of older men, who had the lowest survival, improved the most.
However, multivariable analyses revealed that the effect of period of diagnosis on survival (a lower excess mortality in the more recent time period) disappeared after correction for Breslow thickness in older patients, adding other prognostic variables to the model did not alter the association between period of diagnosis and survival. This confirms that the improved survival in older patients over time can largely be ascribed to the increased detection of thinner melanomas. Contrary, in younger patients changes in Breslow thickness could not explain the lower mortality seen over time. Recent introductions of effective systemic therapies (i.e., BRAF/MEK inhibitors and/or immune checkpoint blockade) may have contributed to an improved survival in younger patients, while older patients less often received those treatments.
Besides thicker melanomas, we also observed that older patients more often had other poor prognostic features. Similar to results of prior studies, elderly were more often diagnosed with nodular melanomas and truncal or head and neck melanomas [Citation6,Citation8,Citation24–26].
Although the gap in survival between younger and older patients is declining, differences still exist. Multiple factors may contribute to a later detection in the older age categories. For example, a lower risk awareness, the lack of a spouse, the presence of comorbidities, cognitive and visual impairments which may complicate early (self-) detection and more nodular melanomas which develop de novo and very quickly. Furthermore, various studies indicated suboptimal staging and/or treatment in older patients which may also have contributed to a poorer prognosis [Citation8,Citation27].
Stage-specific survival indicated that older patients have a worse prognosis compared to their younger counterparts when diagnosed with stage II, III or an unknown stage melanoma at time of diagnosis. A reason for this difference might be the higher recurrence rate and shorter time to recurrences, possibly related to a weakened immune system in older patients, and/or to less optimal staging (understaging) or treatment of older patients [Citation6,Citation27,Citation28].
We did not observe a survival disadvantage for older patients with thin melanomas (stage I) or for patients with distant metastasis at time of diagnosis (stage IV). Both younger and older patients with thin melanomas have a high likelihood of cure, and low rate of local and distant recurrences. Consequently, survival was similar to, or even slightly exceeded, the survival of the general population in both groups. Prognosis of patients with distant metastases (stage IV) was almost equally poor in younger and elderly patients. Therapeutic options were limited for most of those patients, since the vast majority of the patients in our study was diagnosed before new therapies (targeted therapies and immunotherapy) became widely available for advanced stage melanoma.
Our data, however, was limited to stage at diagnosis and we cannot draw conclusions for survival differences in patients with later disease progression.
Details on novel therapies are currently under investigation in the Dutch Melanoma Treatment Registry (DMTR) [Citation29]. While studies have shown that new therapies improved survival [Citation30–34], they are also associated with potentially severe adverse events [Citation32–34]. Considering the large number of older patients and the heterogeneity of the elderly population with regard to frailty and comorbidities, it is of importance to have a representative proportion of elderly patients included in clinical trials, and to monitor how older patients respond to the new modalities. This, to gain knowledge about how they can handle new treatment modalities and benefit optimally from them. First results indicate that elderly less often received new therapies, but that those who are treated show similar responses as their younger counterparts [Citation35,Citation36]. During the latter years of our study period, we indeed saw that with advancing age a lower number of patients with metastatic melanoma (at initial diagnosis) was treated with targeted therapy or immunotherapy. During 2013–2015, 54% (n = 52) of the patients aged <70 years versus 17% (n = 4) when aged ≥80 years (data not shown) was initially treated with one of the new treatment options. Unfortunately, the number of patients was too small for further analyses.
Our study has several limitations. Due to the observational nature of the study, complete information on some major prognostic factors (such as mitotic rate and tumor ulceration) was only available for more recent years, and could not be included in the time trends study. Strengths of this study are its nationwide character (comprehensive full coverage), the large number of patients included, and the complete follow-up with regard to vital status.
Summarized, the gap in incidence of melanoma between younger and elderly patients is increasing while disparities in survival are declining. The most prominent changes over time were seen in older men. They showed the strongest increase in incidence, while their survival improved the most.
The Breslow thickness of elderly patients decreased considerably, explaining the declining gap in survival between the younger and elderly melanoma patients. Nonetheless, elderly melanoma patients still have a survival disadvantage, and their incidence is rising more rapidly compared to younger patients.
The treatment landscape is changing for melanoma patients, also for the elderly. Therefore, it is of importance to stay attentive on melanoma in the elderly to allow them to benefit optimally from those advances.
Supplemental Material
Download (117.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Netherlands Cancer Registry (NCR). Dutch cancer figures; [cited 2018 Dec 3]. Available from: https://www.cijfersoverkanker.nl/.
- World Health Organization. GLOBOCAN 2018; [cited 2019 May 7]. Available from: http://gco.iarc.fr/today/.
- Statistics Netherlands. CBS Statline; [cited 2018 Sep 4]. Available from: https://statline.cbs.nl/Statweb/.
- Arndt V, Sturmer T, Stegmaier C, et al. Patient delay and stage of diagnosis among breast cancer patients in Germany – a population based study. Br J Cancer. 2002;86:1034–1040.
- Dale DC. Poor prognosis in elderly patients with cancer: the role of bias and undertreatment. J Support Oncol. 2003;1:11–17.
- Macdonald JB, Dueck AC, Gray RJ, et al. Malignant melanoma in the elderly: different regional disease and poorer prognosis. J Cancer. 2011;2:538–543.
- Grann AF, Froslev T, Olesen AB, et al. The impact of comorbidity and stage on prognosis of Danish melanoma patients, 1987–2009: a registry-based cohort study. Br J Cancer. 2013;109:265–271.
- Ciocan D, Barbe C, Aubin F, et al. Distinctive features of melanoma and its management in elderly patients: a population-based study in France. JAMA Dermatol. 2013;149:1150–1157.
- Rees MJ, Liao H, Spillane J, et al. Localized melanoma in older patients, the impact of increasing age and comorbid medical conditions. Eur J Surg Oncol. 2016;42:1359–1366.
- Percy C, Van Holten V, Muir C, editors. International classification of diseases for oncology. 2nd ed. Geneva (Switzerland): World Health Organization; 1990.
- Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology. 3rd ed. Geneva (Switzerland): World Health Organization; 2000.
- Sobin LH, Wittekind C. International union against cancer, TNM classification of malignant tumors. 6th ed. New York, NY: Wiley; 2002.
- Sobin LH, Gospodarowicz M, Wittekind C. International union against cancer, TNM classification of malignant tumors. 7th ed. New York, NY: Wiley; 2009.
- Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005;55:231–240.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Ederer FH, H. Instructions to IBM 650 programmers in processing survival computations. Methodological note No. 10. Bethesda MD: National Cancer Institute; 1959.
- Hoejberg L, Gad D, Gyldenkerne N, et al. Trends in melanoma in the elderly in Denmark, 1980–2012. Acta Oncol. 2016;55:52–58.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555–1562.
- Aitken JF, Youlden DR, Baade PD, et al. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995–2014. Int J Cancer. 2017;142:1528–1535
- Haluza D, Simic S, Holtge J, et al. Gender aspects of recreational sun-protective behavior: results of a representative, population-based survey among Austrian residents. Photodermatol Photoimmunol Photomed. 2016;32:11–21.
- Pinault L, Fioletov V. Sun exposure, sun protection and sunburn among Canadian adults. Health Rep. 2017;28:12–19.
- van der Leest RJ, Zoutendijk J, Nijsten T, et al. Increasing time trends of thin melanomas in the Netherlands: what are the explanations of recent accelerations?. Eur J Cancer. 2015;51:2833–2841.
- Kruijff S, Bastiaannet E, Francken AB, et al. Breslow thickness in the Netherlands: a population-based study of 40 880 patients comparing young and elderly patients. Br J Cancer. 2012;107:570–574.
- Reyes-Ortiz CA, Goodwin JS, Freeman JL, et al. Socioeconomic status and survival in older patients with melanoma. J Am Geriatr Soc. 2006;54:1758–1764.
- Lachiewicz AM, Berwick M, Wiggins CL, et al. Survival differences between patients with scalp or neck melanoma and those with melanoma of other sites in the Surveillance, Epidemiology, and End Results (SEER) program. Arch Dermatol. 2008;144:515–521.
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337–343.
- Netherlands Comprehensive Cancer Organisation (IKNL). Kankerzorg in Beeld: de oudere patiënt; 2016.
- Fleming NH, Tian J, Vega-Saenz de Miera E, et al. Impact of age on the management of primary melanoma patients. Oncology. 2013;85:173–181.
- Jochems A, Schouwenburg MG, Leeneman B, et al. Dutch Melanoma Treatment Registry: quality assurance in the care of patients with metastatic melanoma in the Netherlands. Eur J Cancer. 2017;72:156–165.
- Forschner A, Eichner F, Amaral T, et al. Improvement of overall survival in stage IV melanoma patients during 2011–2014: analysis of real-world data in 441 patients of the German Central Malignant Melanoma Registry (CMMR). J Cancer Res Clin Oncol. 2017;143:533–540.
- Leeneman B, Franken MG, Jochems A, et al. Improved survival with ipilimumab in patients with advanced melanoma in real-world clinical practice: first results of the Dutch Melanoma Treatment Registry. Value Health. 2015;18:A440.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516.
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526.
- Chiarion Sileni V, Pigozzo J, Ascierto PA, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J Exp Clin Cancer Res. 2014;33:30.
- Dutch Institute for Clinical Auditing (DICA). Jaarrapportage 2016 [cited 2018 Dec 3]. Available from: https://dica.nl/media/993/DICA-2016-jaarverslag.pdf.