Abstract
Background: Breast cancer patients have a lifelong 2–4-fold increased risk of developing a second primary tumor in the contralateral breast compared with the risk for a first primary breast cancer in the general female population. Prevention of contralateral breast cancer (CBC) has received increased attention during recent decades. Here, we summarize and discuss the available literature on drug preventive therapy and CBC.
Results: The endocrine-targetting drugs, tamoxifen and aromatase inhibitors are used as standard adjuvant treatment for estrogen receptor (ER)-positive breast cancer. Both are associated with relative risk reductions of CBC of up to 50%, but incur serious side effects. Several prescription drugs originally developed for other purposes, including bisphosphonates, statins, non-steroidal anti-inflammatory drugs, metformin, anti-hypertensives and retinoids, have shown anti-cancer activity in preclinical models. However, results of observational studies on CBC are sparse and inconsistent, with only statins demonstrating promise as preventive agents and a potential treatment option for ER-negative breast cancer patients.
Conclusion: Future studies are needed to assess the effect of statins in risk reduction and to identify other drugs with chemopreventive potential against CBC. Eventually, efforts must be directed towards identifying those breast cancer patients likely to benefit most from specific preventive therapies.
Background
Among women diagnosed with breast cancer, the risk of developing a second primary cancer in the contralateral breast is higher than the risk of developing a first primary breast cancer in the general female population [Citation1,Citation2]. The median annual cumulative incidence of contralateral breast cancer (CBC) is 0.5% (range: 0.2–0.7%) [Citation3]. Several factors are associated with increased risk of CBC, including younger age at first breast cancer diagnosis, family history of breast cancer, and lobular histology and estrogen receptor (ER)-negative status of the first breast cancer [Citation4].
Fear of developing CBC motivates some breast cancer patients to undergo contralateral prophylactic mastectomy [Citation5]. This procedure has become more common recently and reduces CBC rates, but evidence of an absolute reduction in CBC risk and survival benefit associated with contralateral prophylactic mastectomy remains uncertain [Citation6]. Other treatment options proven to reduce CBC risk are adjuvant endocrine therapy [Citation7,Citation8] and chemotherapy [Citation7]. Recently, several prescribed drugs, originally developed for other medical conditions, have also shown anti-cancer potential in laboratory and observational studies [Citation9] and may be effective in preventing CBC.
Meta-analyses and reviews have summarized the results from randomized controlled trials (RCTs) and observational studies examining preventive drug therapy for breast cancer. However, the majority of these have focused on the first breast cancer, whereas CBC has received limited attention. One review outlined treatment options to reduce CBC risk, with minor focus on chemopreventive drugs [Citation10]. The purpose of this review is to summarize the available evidence from RCTs and observational studies of drug preventive therapy for CBC, discuss the main limitations of preventive therapy, and propose future research directions.
Adjuvant endocrine breast cancer treatment
Tamoxifen
Tamoxifen is a selective estrogen receptor modulator (SERM) that binds to and blocks the activity of the ER in the breast, while stimulating ER activity in the bone and endometrium [Citation11]. Tamoxifen was the first targeted adjuvant breast cancer therapy and remains the standard endocrine treatment prescribed to premenopausal women with ER-positive breast cancer [Citation12]. Among postmenopausal patients, tamoxifen is an effective alternative treatment among those intolerant to aromatase inhibitors (AIs). Tamoxifen is further approved for primary prevention among high-risk women in the United States (US) and recommended for prevention in United Kingdom (UK) [Citation13]. In addition to reduced breast cancer recurrence and mortality after tamoxifen treatment [Citation14], meta-analyses of RCTs summarized by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) [Citation7,Citation8] and observational studies [Citation15–26] have reported large reductions in the relative risk of CBC (10–56%) among tamoxifen users compared with non-users ( and ). Nonetheless, the absolute risk reductions were small (1.3–4% after 10–15 years) [Citation8,Citation25] ().
Figure 1. Forest plot of studies on preventive drug therapy and risk of contralateral breast cancer. ACE: angiotensin-converting enzyme; AI: aromatase inhibitors; CI: confidence interval; EBCTCG: Early Breast Cancer Trialists’ Collaborative Group; NSAID: non-steroidal anti-inflammatory drug; RCT: randomized controlled trial; TAM: tamoxifen; y: year. aMeta-analysis; bno confidence interval available; conly ER-positive and ER-unknown first breast cancer; dcalendar period 1999–2007; eincludes both new ipsilateral and contralateral breast cancer.
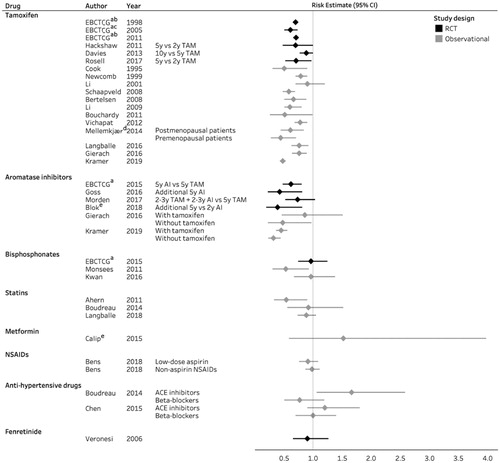
Table 1. Overview of clinical trials and observational studies on the association between tamoxifen and risk of contralateral breast cancer.
The preventive effects of tamoxifen depend on duration of treatment [Citation14,Citation17,Citation25,Citation27–29]. Higher reductions in CBC risk were reported in clinical trials of 5 years of tamoxifen use compared with 1 or 2 years of use [Citation27–29] (). Furthermore, the adjuvant tamoxifen longer against shorter (ATLAS) trial showed a reduced incidence of CBC among women allocated to continue tamoxifen therapy to 10 years rather than stopping at 5 years after breast cancer diagnosis [Citation14]. Observational studies also suggest that tamoxifen is more effective with increasing duration of treatment [Citation17,Citation25].
The preventive effects of tamoxifen persist after discontinuation of treatment, that is, demonstrating a ‘carry-over effect’. Reduced CBC risk estimates up to 5 years after treatment cessation have been reported in clinical trials [Citation8], although observational studies have shown varying lengths of protection after discontinuation of treatment [Citation16,Citation17,Citation25]. Whether the ‘carry-over effect’ is more pronounced with increasing duration of treatment has been evaluated by two studies showing inconsistent results [Citation17,Citation25]. Thus, how duration of tamoxifen affects the length and magnitude of the carry-over effect is a topic deserving further study.
No difference in CBC risk was found according to ER status of the first breast cancer in trials initiated prior to 1990 [Citation27] and in the multi-center WECARE Study [Citation17]. However, a meta-analysis of more recent clinical trials reported reduced CBC risk only among patients with ER-positive first breast cancer [Citation8]. Of concern, some observational studies have reported that tamoxifen use may increase the risk of ER-negative CBC [Citation23,Citation24], but these findings were based on a limited number of exposed cases and not supported by three recent observational studies [Citation17,Citation22,Citation25].
Overall, current evidence indicates that long-term therapy with tamoxifen is more efficient than short-term therapy, and that a ‘carry-over’ effect exists.
Raloxifen
Raloxifen is a second generation SERM, originally developed for prevention of osteoporosis in postmenopausal women [Citation30]. Although raloxifen has demonstrated efficacy in reducing the risk of primary breast cancer, has fewer side effects than tamoxifen, and hence was approved for chemoprevention by the FDA in 2007 [Citation31,Citation32], its effect on CBC risk has not been investigated yet.
Aromatase inhibitors
AIs block the enzyme aromatase, thereby stopping the conversion of androgen to estrogen. In postmenopausal women, estrogen production is primarily driven by the peripheral conversion of androgens, whereas estrogen in premenopausal women is mainly produced in the ovaries [Citation33]. Recent clinical studies have demonstrated that postmenopausal AI therapy, either as single therapy or sequentially with tamoxifen, is more effective than tamoxifen therapy alone, in terms of both prolonged disease-free survival and lower recurrence rates [Citation34]. As a result, third-generation AIs such as anastrozole, letrozole, and exemestane, comprise the ‘state of the art’ preferred adjuvant therapeutic option for postmenopausal women with ER-positive breast cancer [Citation35].
Several, large RCTs have assessed the incidence of CBC in women treated with AIs versus tamoxifen ( and ). A meta-analysis of randomized trials by EBCTCG showed that 5 years of AI treatment was associated with a markedly reduced risk of CBC after 10 years of follow-up compared with 5 years of tamoxifen treatment (RR: 0.62; 95% CI: 0.48–0.80) [Citation34]. Switching to AIs after 2–3 years of tamoxifen for the remainder of the 5-year adjuvant endocrine treatment period was also associated with reduced CBC risk compared with tamoxifen alone (HR: 0.67; 95% CI: 0.51–0.87) [Citation34]. Recently, the results of the 10-year follow-up of the Intergroup Exemestane Study also pointed towards a beneficial effect on CBC associated with switching to AIs [Citation38].
Table 2. Overview over clinical trials and observational studies on the association between aromatase inhibitors and risk of contralateral breast cancer.
There is evidence supporting the benefit of extended AI use beyond the initial 5 years of endocrine therapy from the NCIC Clinical Trial Group (CTG) – MA.17R trial, that extended first-line endocrine therapy with 5 additional years of letrozole versus placebo, and reported a reduced incidence of CBC among women receiving letrozole [Citation37]. In addition, the investigation on the duration of extended adjuvant letrozole treatment (IDEAL) trial found stronger risk reductions for second primary breast cancers for 5 additional years compared with 2.5 additional years of letrozole [Citation40]. In a recent systematic review by Burstein et al., it was stated that ‘a substantial portion of the benefit from extended AI therapy was derived from prevention of second breast cancers’ [Citation43].
Although not recommended in current guidelines, premenopausal women may also benefit from AI therapy when given in combination with ovarian suppression [Citation44]. The suppression of ovarian function trial and tamoxifen and exemestane trial (TEXT) were designed to investigate the efficacy of 5 years of adjuvant exemestane plus ovarian suppression versus adjuvant tamoxifen plus ovarian suppression in premenopausal women with hormone receptor-positive breast cancer. In this study, fewer CBC cases occurred in the exemestane-ovarian suppression group as compared with the tamoxifen-ovarian suppression group [Citation42].
To date, it remains unclear whether one AI is superior over another in CBC prevention. Although the NCIC CTG – MA.27 trial, comparing steroidal (exemestane) versus nonsteroidal (anastrozole) AIs, showed a slightly higher number of CBC cases among women treated with exemestane, future studies are needed before definite conclusions can be drawn [Citation36].
A recent cohort study by Kramer et al. reported more pronounced reductions in CBC risk among patients who received AIs (HR: 0.32, 95% CI: 0.23–0.44) than among those who received tamoxifen (HR: 0.48, 95% CI: 0.44–0.53) when both were compared with no endocrine therapy ( and ) [Citation26]. Previously, the first observational study on AIs by Gierach et al. reported a reduced CBC risk associated with use of AIs without tamoxifen (RR: 0.48, 95% CI: 0.22–0.97); however, not when administered in combination with tamoxifen (RR: 0.86, 95% CI: 0.46–1.51) ( and ) [Citation25]. Moreover, the authors only observed a protective effect of AI against ER-positive CBC [Citation25]. To date, no clinical trials have reported independent risk estimates by hormone receptor status of the contralateral tumor; however, this will be part of the ongoing Long-term Anastrozole versus Tamoxifen Treatment Effects study (NCT01745289). The finding in the study by Gierach et al. [Citation25] is consistent with two trials, investigating first breast cancer risk among unaffected high-risk postmenopausal women, which reported a beneficial effect of AIs only for ER-positive first breast cancer [Citation45,Citation46].
Overall, AI therapy appears to be a good option for the prevention of CBC in postmenopausal breast cancer survivors with studies consistently showing more pronounced CBC risk reductions with AIs than with tamoxifen. Due to the relatively recent introduction of AI therapy in breast cancer patients, issues concerning preventive effects for CBC during treatment according to duration of use and persistence of these effects after discontinuation of AI therapy have not been investigated thoroughly. Future long-term follow-up of RCTs and well-designed observational studies with adequate sample size are needed to answer these questions.
Potentially preventive non-cancer agents
Some drugs initially developed for the prevention and treatment of non-cancer diseases received increased attention due to their potential anti-cancer properties observed in preclinical studies.
Bisphosphonates
Bisphosphonates are the first-line treatment for osteoporosis [Citation47]. They are also effective in the management and prevention of metastatic bone disease and bone loss induced by breast cancer-specific treatments [Citation48–51]. There is a consensus that bisphosphonates should be recommended as part of the adjuvant breast cancer treatment among high-risk postmenopausal women, or among premenopausal women receiving ovarian suppression therapy [Citation52,Citation53]. More recently, several preclinical in vitro and in vivo studies have demonstrated that bisphosphonates may exhibit anti-tumor activities, involving pro-apoptotic and anti-proliferative pathways [Citation54,Citation55]. Moreover, bisphosphonates can indirectly affect the bone marrow microenvironment, the levels of cytokines and growth factors, and tumor angiogenesis [Citation54,Citation55]. The most recent meta-analysis by Ou et al., based on more than 65,000 breast cancer patients from four case-control and four cohort studies, reported a 16% reduction in risk of first primary breast cancer associated with bisphosphonate use (95% CI: 0.77–0.90) [Citation56].
The existing evidence on the association between bisphosphonate use and CBC risk stems from several clinical trials and two observational studies ( and ). In the EBCTCG meta-analysis involving 18,766 breast cancer patients from 26 individual bisphosphonate trials [Citation57], adjuvant bisphosphonate treatment was not associated with a reduced CBC risk (RR: 0.96, 95% CI: 0.74–1.25) [Citation57]. The majority of patients included in these trials had ER-positive first breast cancer and were accordingly treated with tamoxifen or AI. Therefore, a potential association with use of bisphosphonates may have been masked by the established CBC preventive effects of endocrine therapy.
Table 3. Overview of clinical trials and observational studies on the association between non-cancer drugs and risk of contralateral breast cancer.
A nested case-control study including 351 CBC cases and 662 controls, showed that ever use of any bisphosphonate was associated with a substantial reduction in CBC (OR: 0.53, 95% CI: 0.30–0.93) [Citation58]. Furthermore, the risk decreased with increasing duration of bisphosphonate use among current users (per year: OR: 0.71, 95% CI: 0.52–0.96) [Citation58]. In contrast, a more recent US cohort study did not support an inverse association between bisphosphonate use and CBC risk (HR: 0.96, 95% CI: 0.67–1.38) [Citation59]. Both observational studies were conducted solely among hormone receptor-positive breast cancer patients, and the question remains whether bisphosphonates could affect CBC development in hormone receptor-negative breast cancer patients.
Statins
Statins (HMG-CoA reductase inhibitors) are cholesterol-lowering drugs that are effective in preventing cardiovascular disease [Citation69]. Preclinical studies of breast cancer cell lines and animal models have suggested that statins also impede tumor initiation, growth, and metastasis [Citation70–72]. In particular, the lipophilic statins have shown stronger anti-neoplastic effects in ER-negative cell lines [Citation71,Citation73]. However, meta-analyses of RCTs and observational studies investigating overall statin use and incidence of first breast cancer have consistently reported neutral associations [Citation74–77]. The most recent meta-analysis from 2017 [Citation77] included sub-group analyses by statin solubility and duration of use, and suggested a reduced breast cancer risk associated with long-term use of any statin (RR: 0.74; 95% CI: 0.52–1.04) and the highly lipophilic statin, simvastatin (RR: 0.90; 95% CI: 0.80–1.01). The few breast cancer incidence studies, specifically investigating lipophilic statins, overall [Citation78] or by duration [Citation79] according to ER status reported associations close to unity.
To date, only three cohort studies have examined the association between statin use and risk of CBC [Citation60–62] ( and ). The first study including 18,769 women diagnosed with primary breast cancer from the Danish Breast Cancer Group (DBCG) database reported a 46% reduced risk of CBC (95% CI: 0.33–0.90) associated with use of simvastatin [Citation60]. The second study included 4216 women from the US with primary breast cancer and had limited statistical precision [Citation61]. The most recent study, including 52,723 breast cancer patients from the DBCG database within an extended period, presented detailed information on timing, type of statins, and patterns of use as well as ER status [Citation62]. In this study, ever use of statins was associated with a slight reduction in CBC risk (HR: 0.88; 95% CI: 0.73–1.05) together with a more marked risk reduction for long-term statin use (HR: 0.64; 95% CI: 0.43–0.96). However, this risk reduction was not seen among long-term high intensity users or consistent users. A substantial reduction in CBC risk was observed among patients diagnosed with ER-negative first breast cancer (HR: 0.67; 95% CI: 0.45–1.00).
Thus, current evidence on statins and CBC risk is sparse, but there is a suggestion of a reduced CBC risk associated with statin use [Citation60,Citation62], particularly among patients diagnosed with ER-negative breast cancer [Citation62]. Future observational studies are warranted to establish whether statins could be useful for preventing a new CBC, especially among women with ER-negative disease.
Metformin
Metformin, a widely used oral antidiabetic drug, has received substantial interest as a potential anti-cancer agent [Citation80]. Preclinical studies suggest that metformin inhibits the proliferation of breast cancer cells and delays the development of mammary tumors in mice [Citation81]. This contrasts to the anti-diabetic drug insulin, which has been found to increase breast cancer cell proliferation [Citation82]. Recent meta-analyses of observational studies by Col et al. [Citation83] and Zhang et al. [Citation84] reported breast cancer risk reductions of 17% (95% CI: 0.71–0.97) and 6% (95% CI: 0.91–0.98), respectively, associated with metformin use among women without prior breast cancer.
Yet, only two studies have investigated the influence of metformin use on second breast cancer events including CBC ( and ). A US cohort study by Oppong et al. including breast cancer patients with type II diabetes reported lower CBC incidence among metformin users compared with non-users. However, the study was too small to provide an appropriate risk estimate with only seven CBC cases occurring during follow-up [Citation63]. A larger US cohort study by Calip et al. did not observe an association between metformin use and risk of a second primary breast cancer including both contralateral and ipsilateral cases [Citation64]. Any cancer preventive effects of metformin might be masked if used in combination with insulin. In the available studies, Calip et al. adjusted for insulin in their multivariable model [Citation64], while in the study by Oppong et al. the proportion of combined users was small [Citation63]. The ongoing phase III study (NCIC CTG – MA.32 trial) investigating the effect of metformin versus placebo in addition to standard adjuvant therapy, may provide further knowledge on the association between metformin use and CBC risk.
Non-steroidal anti-inflammatory drugs (NSAIDs)
Aspirin and non-aspirin NSAIDs, including traditional nonselective NSAIDs and cyclooxygenase (COX)-2 inhibitors, are widely prescribed drugs with cancer preventive properties. These drugs inhibit the activity of the COX enzymes, which are important in platelet function and in the production of inflammatory prostaglandins [Citation85]. Various in vitro and in vivo studies have shown that aspirin and non-aspirin NSAIDs inhibit the proliferation of breast cancer cells and suppress tumor growth [Citation86,Citation87]. The most recent meta-analyses reported slightly decreased breast cancer risk associated with aspirin [Citation88,Citation89] and in one of these with COX-2 inhibitors [Citation88], but not with overall non-aspirin NSAID use [Citation88].
The effect of aspirin and other NSAID use on CBC risk has been studied in two Danish cohort studies ( and ). Among 52,723 breast cancer patients, HRs for CBC of 0.91 (95% CI: 0.76–1.09) and 0.98 (95% CI: 0.87–1.11) were observed for low-dose aspirin (75–150 mg) and non-aspirin NSAID use, respectively [Citation65,Citation66]. The ongoing Add-Aspirin trial (NCT02804815), assessing the efficacy of adjuvant aspirin (100 mg or 300 mg versus placebo) in breast cancer patients, may provide further understanding of the potential of aspirin in prevention of CBC among breast cancer survivors.
Antihypertensive medications
Beta-blockers (β-blockers), angiotensin converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) are widely used drugs in the treatment of hypertension and heart disease. Laboratory studies of human cancer cell lines and animal models have demonstrated anti-proliferative and anti-angiogenic properties of propranolol, a nonselective β-adrenergic receptor antagonist [Citation90]. ACE-inhibitors and ARBs may exert anti-neoplastic effects by inhibiting the renin-angiotensin system and its downstream signaling proteins such as vascular endothelial growth factor and the transcription factor, NFκB [Citation91,Citation92]. Despite the promising laboratory results, observational studies on risk of first breast cancer with anti-hypertensive use have provided mixed results [Citation93].
Two US-based observational studies investigated the association between anti-hypertensive use and CBC risk ( and ). Both studies were small including 143 and 352 CBC cases, respectively [Citation61,Citation67]. The first study based on a cohort of 4,216 breast cancer patients suggested a decreased risk of CBC associated with β-blocker use (HR: 0.77; 95% CI: 0.50–1.19), whereas ACE-inhibitors use was associated with an increased risk of CBC (HR: 1.66, 95% CI: 1.06–2.58) [Citation61]. The second study [Citation67] reported no reduced risk with use of β-blockers (OR: 1.0, 95% CI: 0.7–1.4), while the association for ACE inhibitors (OR: 1.2, 95% CI: 0.9–1.8) was consistent with the first study.
Retinoids
Fenretinide, a synthetic derivative of retinoic acid, inhibits breast cancer development in animal models [Citation94]. In an RCT, Veronesi et al. assessed the efficacy of 5-year treatment with fenretinide in preventing second breast malignancies in women with early breast cancer () (). Overall, no reduction in CBC risk was observed in a post-intervention study with a median follow-up of 14.6 years (HR: 0.90, 95% CI: 0.65–1.26) [Citation68]. A beneficial effect of fenretinide on CBC risk was, however, suggested in premenopausal women (HR: 0.63, 95% CI: 0.38–1.04) irrespective of the hormone receptor status of the first breast cancer [Citation68].
Discussion
Short summary of the results
Research in the field of preventive therapy for breast cancer has made important discoveries during the past decades; however, CBC has received limited focus. In this review, we summarized the available literature regarding the association between various drugs and CBC risk among breast cancer patients. To date, the most favorable candidates for prevention of CBC are the endocrine therapies, tamoxifen, and AIs. Widespread use of these drugs among patients with ER-positive first breast cancer has possibly led to declining CBC rates [Citation95], whereas limited therapeutic options, and thus CBC prevention, are available for patients with ER-negative first breast cancer.
Various drugs, originally developed for other purposes, have shown preventive effects against breast cancer in laboratory and/or observational studies. Among these, statins show perhaps the most promising results supporting a reduction in CBC risk [Citation60,Citation62]. Moreover, mounting evidence supports reduced mortality [Citation96] and risk of recurrence [Citation96,Citation97] associated with statin use following a breast cancer diagnosis. A number of pilot trials (planned or ongoing) are currently listed at www.clinicaltrials.gov with the primary aim of investigating the effect of statins on several breast cancer biomarkers [Citation72]. However, before statins can be approved for use in breast cancer patients, clinical trials in the adjuvant setting for breast cancer are needed [Citation70]. There is some evidence supporting a reduced CBC risk associated with use of the other drugs summarized in this review; however, more studies are needed to explore these further.
RCTs versus observational studies
The evidence for potential preventive drug effects against cancer comes from two main types of studies, RCTs and observational studies. Randomized trials are often considered the optimal design; however, each study type has their own strengths and weaknesses. Randomization in trials ensures similarities between groups with respect to both measured and unmeasured confounding factors. Theoretically, study groups only differ in the type of treatment they receive, allowing causal conclusions to be drawn. On the other hand, RCTs often apply strict in- and exclusion criteria. Thus, a trial population is selected and may have limited generalizability. Data from clinical trials on CBC risk are only available for tamoxifen, AIs, and bisphosphonates. Although the definition of recurrences often includes new contralateral tumors, some of the trials investigating breast cancer recurrence did not report separate risk estimates for CBC, or had limited information on tumor characteristics of the contralateral tumors. Moreover, many trials had limited study size and length of follow-up, and were thus underpowered to provide meaningful CBC risk estimates. Observational studies can be valuable in examining the effects of preventive drugs on long-term outcomes in large human populations. However, these studies are prone to confounding, particularly confounding by indication as well as other drug exposures, lifestyle or socio-economic factors that are often challenging to account for. As for RCTs, a limited number of observational studies on recurrences focused specifically on the association with CBC. Results from RCTs and observational studies can be difficult to compare as duration of use and definitions of current and past use often vary. However, when large datasets are available, observational studies can also examine various levels of duration and time since last use. Thus, although RCTs are the gold standard for establishing causal conclusions, observational studies provide much needed information on the preventive effects in real-life settings.
Limitations of drug preventive therapy
Preventive drug therapy, while promising, is also not without limitations. First, the efficacy of tamoxifen in reducing recurrence, mortality, and CBC risk is highly dependent on patient adherence. The most recent systematic review reported discontinuation rates of up to 73% at the end of 5 years of endocrine treatment [Citation98]. A small UK trial observed high compliance rates for bisphosphonate treatment with more than 90% of the breast cancer patients taking their monthly doses [Citation99]. Although not investigated specifically in breast cancer patients, studies on statin adherence have provided inconsistent results [Citation100–102]. The data sources used to define adherence differ in the literature, from self-report by patients, drug concentration assessment in serum, to medical records review. Adherence is influenced by multiple factors such as patient, health and physician-related factors, and side effects. In contrast to observational studies, breast cancer patients enrolled in clinical trials are often highly motivated to participate and are closely monitored, which positively influences adherence. Therefore, the drug effects seen in RCTs may not be replicated in real-life observational studies simply due to lower adherence.
Breast cancer patients treated with tamoxifen or AIs suffer from side effects ranging from relatively mild menopausal symptoms to more severe adverse events. Side effects are also seen for non-cancer drugs. Detailed information on side effects of the drugs discussed in this review is presented in the Supplementary Material.
The use of preventive drugs should be considered individually by balancing their benefits and toxicity. For most breast cancer patients, the absolute risk of CBC is low. Consequently, the large reductions in relative risks of CBC observed for adjuvant endocrine therapy can be misleading, since the absolute risk reductions are small. In the clinical guidelines for breast cancer adjuvant endocrine therapy, the primary consideration is risk of recurrence. For patients at low-risk of recurrence, currently not treated with adjuvant therapy [Citation103], use of endocrine therapy may not be justified on the benefits of CBC prevention alone. Thus, it is important to identify subgroups of women with breast cancer at high-risk of CBC, who are likely to benefit most from preventive therapy.
Personalized preventive therapy
Future studies should focus on developing risk prediction models to identify high-risk patients [Citation104]. In that context, we need to identify biomarkers that could be included in these models to more accurately predict the risk of CBC. Biomarkers may ultimately be used to predict whether a woman will benefit from a specific treatment or not thereby personalizing preventive therapy for breast cancer survivors. Women receiving such personalized treatment may also have a greater willingness to accept possible side effects related to preventive drugs.
In this review, we primarily focused on preventive drugs and CBC development among breast cancer patients, but CBC has been proposed to serve as a surrogate marker for development of first primary breast cancer. For example, the first evidence for tamoxifen as a preventive agent in healthy women at high-risk of breast cancer originates from trials of breast cancer patients reporting reduced incidence of contralateral breast tumors [Citation105]. Therefore, a favorable effect on CBC risk would provide a strong rationale for the effectiveness of a preventive drug in unaffected women at high-risk of first primary breast cancer.
Conclusion
Major progress has been achieved in the prevention of CBC mainly by incorporating adjuvant endocrine therapy with tamoxifen and AIs into routine clinical practice. Nevertheless, these drugs are associated with serious side effects and are not indicated for patients with ER-negative first breast cancer. At present, the effectiveness of other non-cancer agents in reducing CBC risk is limited. Yet, statins show promise, have fewer, and less severe adverse effects than endocrine therapies, and may be a successful treatment option for ER-negative breast cancer patients. For all preventive drugs, important questions regarding the optimal duration and dosing schedule require further investigation. Furthermore, better understanding of the anti-cancer mechanisms demonstrated in experimental studies and the identification of biomarkers will help us move forward towards more personalized medicine.
Supplemental Material
Download MS Word (35.8 KB)Disclosure statement
Lene Mellemkjaer has an immediate family member employed at Novo Nordisk, and has an immediate family member who owns stocks in Novo Nordisk. All other authors have declared no conflicts of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Curtis RE, Ron E, Hankey BF, et al. New malignancies following breast cancer. In: Curtis RE, Freedman M, Ron E, et al., editors. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. Bethesda (MD): National Institutes of Health (NIH); 2006. p. 181–205.
- Evans HS, Lewis CM, Robinson D, et al. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84:435–440.
- Spronk I, Schellevis FG, Burgers JS, et al. Incidence of isolated local breast cancer recurrence and contralateral breast cancer: a systematic review. The Breast. 2018;39:70–79.
- Narod SA. Bilateral breast cancers. Nat Rev Clin Oncol. 2014;11:157–166.
- Yao K, Sisco M, Bedrosian I. Contralateral prophylactic mastectomy: current perspectives. Int J Women's Health. 2016;8:213–223.
- Fayanju OM, Stoll CR, Fowler S, et al. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg. 2014;260:1000–1010.
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717.
- Early Breast Cancer Trialists' Collaborative Group, Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784.
- Cuzick J. Preventive therapy for cancer. Lancet Oncol. 2017;18:e472–e482.
- Davies KR, Cantor SB, Brewster AM. Better contralateral breast cancer risk estimation and alternative options to contralateral prophylactic mastectomy. Int J Women's Health. 2015;7:181–187.
- Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53.
- Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: summary of the consensus discussion. Breast Care (Basel). 2011;6:136–141.
- Cuzick J. Progress in preventive therapy for cancer: a reminiscence and personal viewpoint. Br J Cancer. 2018;118:1155–1161.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816.
- Bertelsen L, Bernstein L, Olsen JH, et al. Effect of systemic adjuvant treatment on risk for contralateral breast cancer in the Women's Environment, Cancer and Radiation Epidemiology Study. J Natl Cancer Inst. 2008;100:32–40.
- Cook LS, Weiss NS, Schwartz SM, et al. Population-based study of tamoxifen therapy and subsequent ovarian, endometrial, and breast cancers. J Natl Cancer Inst. 1995;87:1359–1364.
- Langballe R, Mellemkjaer L, Malone KE, et al. Systemic therapy for breast cancer and risk of subsequent contralateral breast cancer in the WECARE Study. Breast Cancer Res. 2016;18:65.
- Mellemkjaer L, Steding-Jessen M, Frederiksen K, et al. Risk of contralateral breast cancer after tamoxifen use among Danish women. Ann Epidemiol. 2014;24:843–848.
- Newcomb PA, Solomon C, White E. Tamoxifen and risk of large bowel cancer in women with breast cancer. Breast Cancer Res Treat. 1999;53:271–277.
- Schaapveld M, Visser O, Louwman WJ, et al. The impact of adjuvant therapy on contralateral breast cancer risk and the prognostic significance of contralateral breast cancer: a population based study in the Netherlands. Breast Cancer Res Treat. 2008;110:189–197.
- Vichapat V, Garmo H, Holmqvist M, et al. Tumor stage affects risk and prognosis of contralateral breast cancer: results from a large Swedish-population-based study. J Clin Oncol. 2012;30:3478–3485.
- Bouchardy C, Benhamou S, Fioretta G, et al. Risk of second breast cancer according to estrogen receptor status and family history. Breast Cancer Res Treat. 2011;127:233–241.
- Li CI, Daling JR, Porter PL, et al. Adjuvant hormonal therapy for breast cancer and risk of hormone receptor-specific subtypes of contralateral breast cancer. Cancer Res. 2009;69:6865–6870.
- Li CI, Malone KE, Weiss NS, et al. Tamoxifen therapy for primary breast cancer and risk of contralateral breast cancer. J Natl Cancer Inst. 2001;93:1008–1013.
- Gierach GL, Curtis RE, Pfeiffer RM, et al. Association of adjuvant tamoxifen and aromatase inhibitor therapy with contralateral breast cancer risk among us women with breast cancer in a general community setting. JAMA Oncol. 2017;3:186–193.
- Kramer I, Schaapveld M, Oldenburg HSA, et al. The influence of adjuvant systemic regimens on contralateral breast cancer risk and receptor subtype. J Natl Cancer Inst. 2019;111:709–718.
- Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467.
- Hackshaw A, Roughton M, Forsyth S, et al. Long-term benefits of 5 years of tamoxifen: 10-year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. J Clin Oncol. 2011;29:1657–1663.
- Rosell J, Nordenskjold B, Bengtsson NO, et al. Long-term effects on the incidence of second primary cancers in a randomized trial of two and five years of adjuvant tamoxifen. Acta Oncol. 2017;56:614–617.
- Gizzo S, Saccardi C, Patrelli TS, et al. Update on raloxifene: mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstetrical Gynecol Surv. 2013;68:467–481.
- Waters EA, McNeel TS, Stevens WM, et al. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134:875–880.
- Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: preventing breast cancer. Cancer Prev Res (Philadelphia, Pa). 2010;3:696–706.
- Schneider R, Barakat A, Pippen J, et al. Aromatase inhibitors in the treatment of breast cancer in post-menopausal female patients: an update. Breast Cancer Targets Ther. 2011;3:113–125.
- Early Breast Cancer Trialists' Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352.
- Jensen MB, Laenkholm AV, Offersen BV, et al. The clinical database and implementation of treatment guidelines by the Danish Breast Cancer Cooperative Group in 2007-2016. Acta Oncol. 2018;57:13–18.
- Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27 – a randomized controlled phase III trial. J Clin Oncol. 2013;31:1398–1404.
- Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209–219
- Morden JP, Alvarez I, Bertelli G, et al. Long-term follow-up of the Intergroup Exemestane Study. J Clin Oncol. 2017;35:2507–2514.
- Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol. 2017;18:1502–1511.
- Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL Trial (BOOG 2006-05). J Natl Cancer Inst. 2018;110:40–48.
- Colleoni M, Luo W, Karlsson P, et al. Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:127–138.
- Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–137
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37:423–438.
- Carlson RW, Theriault R, Schurman CM, et al. Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor–positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol. 2010;28:3917–3921.
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391.
- Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–1048.
- Black DM, Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2016;374:254–262.
- Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997–1006.
- Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29:1221–1227.
- Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013;24:398–405.
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593.
- Hadji P, Coleman RE, Wilson C, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016;27:379–390.
- Danish Breast Cancer Cooperative Group. Medicinsk behandling; 2016 [cited 2019 Jul 17]. Available from: http://www.dbcg.dk/
- Morgan G, Lipton A. Antitumor effects and anticancer applications of bisphosphonates. Semin Oncol. 2010;37:S30–S40.
- Holen I, Coleman RE. Anti-tumour activity of bisphosphonates in preclinical models of breast cancer. Breast Cancer Res. 2010;12:214
- Ou YJ, Chiu HF, Wong YH, et al. Bisphosphonate use and the risk of breast cancer: a meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2017;26:1286–1295.
- Early Breast Cancer Trialists’ Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361.
- Monsees GM, Malone KE, Tang M-T, et al. Bisphosphonate use after estrogen receptor–positive breast cancer and risk of contralateral breast cancer. J Natl Cancer Inst. 2011;103:1752–1760.
- Kwan ML, Shi JM, Habel LA, et al. Effectiveness of bisphosphonate use and risk of contralateral breast cancer and recurrence in women with early-stage breast cancer treated with tamoxifen. Breast Cancer Res Treat. 2016;156:379–389.
- Ahern TP, Pedersen L, Tarp M, et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103:1461–1468
- Boudreau DM, Yu O, Chubak J, et al. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat. 2014;144:405–416.
- Langballe R, Cronin-Fenton D, Dehlendorff C, et al. Statin use and risk of contralateral breast cancer: a nationwide cohort study. Br J Cancer. 2018;119:1297–1305.
- Oppong BA, Pharmer LA, Oskar S, et al. The effect of metformin on breast cancer outcomes in patients with type 2 diabetes. Cancer Med. 2014;3:1025–1034.
- Calip GS, Yu O, Hoskins KF, et al. Associations between diabetes medication use and risk of second breast cancer events and mortality. Cancer Causes Control. 2015;26:1065–1077.
- Bens A, Friis S, Dehlendorff C, et al. Low-dose aspirin use and risk of contralateral breast cancer: a Danish nationwide cohort study. Prev Med. 2018;116:186–193
- Bens A, Cronin-Fenton D, Dehlendorff C, et al. Non-aspirin NSAIDs and contralateral breast cancer risk. Int J Cancer. 2018;144:1243–1250.
- Chen L, Malone KE, Li CI. Use of antihypertensive medications not associated with risk of contralateral breast cancer among women diagnosed with estrogen receptor-positive invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2015;24:1423–1426.
- Veronesi U, Mariani L, Decensi A, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17:1065–1071
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;316:2008–2024.
- Ahern TP, Lash TL, Damkier P, et al. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol. 2014;15:e461–8.
- Campbell MJ, Esserman LJ, Zhou Y, et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–8714.
- Borgquist S, Bjarnadottir O, Kimbung S, et al. Statins – a role in breast cancer therapy?. J Intern Med. 2018;284:346–357.
- Vinayak S, Kurian AW. Statins may reduce breast cancer risk, particularly hormone receptor-negative disease. Curr Breast Cancer Rep. 2009;1:148–156.
- Bonovas S, Filioussi K, Tsavaris N, et al. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23:8606–8612.
- Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat. 2012;135:261–269.
- Wu QJ, Tu C, Li YY, et al. Statin use and breast cancer survival and risk: a systematic review and meta-analysis. Oncotarget. 2015;6:42988–43004.
- Islam MM, Yang HC, Nguyen PA, et al. Exploring association between statin use and breast cancer risk: an updated meta-analysis. Arch Gynecol Obstet. 2017;296:1043–1053
- Borgquist S, Tamimi RM, Chen WY, et al. Statin use and breast cancer risk in the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev. 2016;25:201–206.
- Woditschka S, Habel LA, Udaltsova N, et al. Lipophilic statin use and risk of breast cancer subtypes. Cancer Epidemiol Biomarkers Prev. 2010;19:2479–2487.
- Leone A, Di Gennaro E, Bruzzese F, et al. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355–376.
- Thompson AM. Molecular pathways: preclinical models and clinical trials with metformin in breast cancer. Clin Cancer Res. 2014;20:2508–2515.
- Chappell J, Leitner JW, Solomon S, et al. Effect of insulin on cell cycle progression in MCF-7 breast cancer cells. Direct and potentiating influence. J Biol Chem. 2001;276:38023–38028.
- Col NF, Ochs L, Springmann V, et al. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135:639–646.
- Zhang P, Li H, Tan X, et al. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37:207–218.
- Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11:81s–110s
- Maity G, De A, Das A, et al. Aspirin blocks growth of breast tumor cells and tumor-initiating cells and induces reprogramming factors of mesenchymal to epithelial transition. Lab Invest. 2015;95:702–717.
- Dai ZJ, Ma XB, Kang HF, et al. Antitumor activity of the selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer in vitro and in vivo. Cancer Cell Int. 2012;12:53.
- de Pedro M, Baeza S, Escudero MT, et al. Effect of COX-2 inhibitors and other non-steroidal inflammatory drugs on breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2015;149:525–536.
- Lu L, Shi L, Zeng J, et al. Aspirin as a potential modality for the chemoprevention of breast cancer: a dose-response meta-analysis of cohort studies from 857,831 participants. Oncotarget 2017;8:40389–40401.
- Pasquier E, Ciccolini J, Carre M, et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2:797–809.
- Napoleone E, Cutrone A, Cugino D, et al. Inhibition of the renin-angiotensin system downregulates tissue factor and vascular endothelial growth factor in human breast carcinoma cells. Thrombosis Res. 2012;129:736–742.
- Du N, Feng J, Hu LJ, et al. Angiotensin II receptor type 1 blockers suppress the cell proliferation effects of angiotensin II in breast cancer cells by inhibiting AT1R signaling. Oncol Rep. 2012;27:1893–1903.
- Ni H, Rui Q, Zhu X, et al. Antihypertensive drug use and breast cancer risk: a meta-analysis of observational studies. Oncotarget. 2017;8:62545–62560.
- Garattini E, Bolis M, Garattini SK, et al. Retinoids and breast cancer: from basic studies to the clinic and back again. Cancer Treatment Rev. 2014;40:739–749.
- Nichols HB, Berrington de Gonzalez A, Lacey JV, Jr, et al. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29:1564–1569.
- Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: a systematic review and meta-analysis. Int J Cancer. 2016;139:1281–1288.
- Borgquist S, Giobbie-Hurder A, Ahern TP, et al. Cholesterol, cholesterol-lowering medication use, and breast cancer outcome in the BIG 1-98 Study. J Clin Oncol. 2017;35:1179–1188.
- Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134:459–478.
- Lester JE, Dodwell D, Purohit OP, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res. 2008;14:6336–6342.
- Svensson E, Nielsen RB, Hasvold P, et al. Statin prescription patterns, adherence, and attainment of cholesterol treatment goals in routine clinical care: a Danish population-based study. Clin Epidemiol. 2015;7:213–223.
- Perreault S, Blais L, Lamarre D, et al. Persistence and determinants of statin therapy among middle-aged patients for primary and secondary prevention. Br J Clin Pharmacol. 2005;59:564–573.
- Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep. 2013;15:291.
- Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–1060.
- Chowdhury M, Euhus D, Onega T, et al. A model for individualized risk prediction of contralateral breast cancer. Breast Cancer Res Treat. 2017;161:153–160.
- Cuzick J, Baum M. Tamoxifen and contralateral breast cancer. Lancet. 1985;2:282.
