Abstract
Purpose: This study aimed to evaluate whether an early beginning of the adjuvant stereotactic radiotherapy after macroscopic complete resection of 1–3 brain metastases is essential or whether longer intervals between surgery and radiotherapy are feasible.
Material and methods: Sixty-six patients with 69 resection cavities treated with HFSRT after macroscopic complete resection of 1–3 brain metastases between 2009 and 2016 in our institution were included in this study. Overall survival, local recurrence and locoregional recurrence were evaluated depending on the time interval from surgery to the start of radiation therapy.
Results: Patients that started radiotherapy within 21 days from surgery had a significantly decreased OS compared to patients treated after a longer interval from surgery (p < .01). There was no significant difference between patients treated ≥ 34 and 22–33 days from surgery (p = .210). In the univariate analysis, local control was superior for patients starting treatment 22–33 days from surgery compared to a later start (p = .049). This effect did not prevail in a multivariate model. There was no significant difference between patients treated within 21 days and patients treated more than 33 days after surgery (p = .203). Locoregional control was not influenced by RT timing (p = .508).
Conclusion: A short delay in the start of radiotherapy does not seem to negatively impact the outcome in patients with resected brain metastases. We even observed an unexpected reduction in OS in patients treated within 21 days from surgery. Further studies are needed to define the optimal timing of postoperative radiotherapy to the resection cavity.
Introduction
Around 20–40% of cancer patients develop brain metastases in the course of their disease converting them into the most common intracerebral tumor in adults [Citation1]. Approximately, 40–50% of these patients suffer from a single lesion at the time of diagnosis [Citation2]. Surgical resection is an important pillar in the treatment of patients with large and symptomatic brain metastases and a limited number of lesions. Since 2-year local recurrence rates reach 60% without further therapy even after total resection, adjuvant radiotherapy is the standard of care [Citation3]. To avoid the negative effect of whole brain radiotherapy on cognitive functioning, local treatment options are preferred [Citation4,Citation5]. Radiosurgery and hypofractionated stereotactic radiotherapy (HFRSRT) have both shown excellent local control rates [Citation6]. While the ideal target volume and dosing schemes are under constant investigation, little is known about the role the timing of adjuvant radiotherapy (RT) plays in the treatment of resected brain metastases.
The interval between surgery and adjuvant RT has been shown to influence the outcome of treatment differently, depending on the tumor entity in question. Delayed RT seems to negatively impact local control in patients with head & neck and breast cancer. For primary tumors of the brain, namely glioblastoma multiforme, and high-grade pediatric glioma, no disadvantage could be observed for patients that received delayed RT [Citation7–10]. In the irradiation of resected brain metastases local changes occurring in the brain after surgery, as well as, different tumor cell biologies are likely to play a role in their response to the timing of adjuvant RT.
This study aimed to evaluate whether an early beginning of the adjuvant RT after resection of 1–3 brain metastases is essential or whether longer intervals between surgery and RT are feasible.
Material and methods
Patients
Sixty-six patients with 69 resection cavities treated with HFSRT after macroscopic complete resection of 1–3 brain metastases between 2009 and 2016 in our institution were included in this study. Patients with incomplete resection suspected intraoperatively or on postoperative MRI and patients with prior whole brain radiotherapy were excluded from the study. Median age was 66 years (range 19–85 years), most common primaries were non-small cell lung cancer (19 cases/28.8%); gastrointestinal cancers (14 cases/21.2%) and breast cancer (11 cases/16.7%). Patients’ characteristics are shown in . All patients were treated by the Declaration of Helsinki. A written informed consent in the use of scientific data was obtained by all patients. This study was approved by the Ethics Committee of the Technical University of Munich.
Table 1. Patient characteristics in the treatment groups.
Radiotherapy
35 Gy were applied in daily doses of 5 Gy. Dose prescription was to the 95–100% isodose line. The clinical target volume (CTV) was defined as the resection cavity (encompassing residual tumor, if present) plus a safety margin of 2–3 mm. Planning target volume (PTV) was generated with an additional margin of 1–2 mm to the CTV. Treatment planning was carried out using iPlan RT Dose treatment planning software (BrainLAB AG, Munich, Germany) or Eclipse software (version 13; Varian Medical Systems, Palo Alto, CA, USA).
Irradiation was performed with a Clinac Trilogy linear accelerator equipped with a 120 HD multi-leaf collimator (Varian Medical Systems, Palo Alto, CA, USA) and 6 MV photons. Further metastases were treated with simultaneous or sequential stereotactic radiosurgery with a dose of 20 Gy prescribed to the 80%-Isodose line or hypofractionated RT with 35 (or 30 Gy, if adjacent to brain stem) in daily doses of 5 Gy. A high precision treatment set-up was applied using a frameless thermoplastic mask system (BrainLAB AG, Munich, Germany). Daily image-guided radiotherapy (IGRT) was performed with the ExacTrac stereoscopic X-ray imaging system.
Timing of radiotherapy
In our department, there were no specifications determining the time interval between surgery and RT or a selection regarding RT scheduling based on known prognostic factors. Generally, RT is initiated in a timely fashion after recovery from surgery, and wound healing is completed. However, often patients wish to recover at home for a certain time or take part in a rehabilitation measure and therefore postpone RT. The timing of RT was defined as the number of days after surgery. Patients were categorized in 3 equal groups: ≤21 days from surgery, 21–33 days after surgery and ≥34 days after surgery.
Statistical evaluation
Local recurrence and loco-regional recurrence was calculated from the day of surgery until the date of tumor recurrence. Local recurrence was defined as a recurrence at the site of the initial metastases; locoregional recurrence also includes distant brain recurrence. Recurrence was documented if stated as such in the MRI report. In patients with more than one resection cavity, each cavity was regarded individually in the calculation of local recurrence. For the evaluation of overall survival (OS), the time interval between surgery to the date of death or the last contact was calculated. In order to reduce the selection bias produced by calculating survival from the date of surgery, we performed a second evaluation excluding deaths occurring within 2 months from surgery. Two months was chosen as the cutoff as this was approximately the mean interval between surgery and radiotherapy in the group with ≥34 days from surgery. This applied to 2 patients treated within 21 days from surgery.
Continuous data were expressed as means ± standard deviation (SD) or median and range, categorical data as frequency counts or percentages. OS and recurrence rates were calculated by Kaplan–Meier-method. For comparison of survival distributions, the log-rank test was used. The Cox proportional hazards model was applied to test for the independent effect of RT timing after adjusting for confounding factors. Confounding factors we adjusted for were age, Karnofsky performance score (KPS), recursive partitioning analysis (RPA), histology, year of RT and number of metastases. The influence of local recurrence between RT and surgery was individually assessed but not adjusted for in a multivariate model.
Categorical data were compared by chi-square test. A p value of 0.05 was defined as the threshold for statistical significance within a confidence interval of 95%. All calculations and figures were done with the software packages SPSS 23 (IBM, Armonk, NY, USA).
Results
Timing of radiotherapy
Mean time between surgery and beginning of RT was 32.5 days (±23.8 days). Mean time between surgery and RT decreased over time. In 2009–2011, 2012–2014 and 2015–2016, it was 43.6 (±43.7) days, 33.8 (±15.1) days and 23.7 (±8.2) days, respectively (p = .037). In 10.6% of cases, RT was delayed due to patient wish. 3% of delays were caused by capacity shortages or organizational reasons. In 86.4% of cases, no specific reason for a certain starting date of RT could be retrieved.
Local recurrence before radiotherapy
The time between surgery and RT is significantly correlated with local recurrence (p = .043) until the start of RT. Local recurrence before RT did not significantly influence OS (p = .331). The influence of local recurrence between surgery and RT on local control after RT was not significant (p = .322). Locoregional control was not affected by local recurrence (p = .463) before RT.
Overall survival
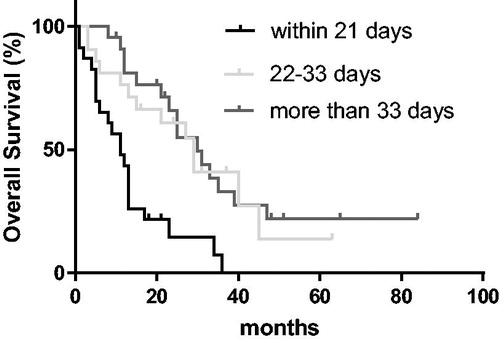
Mean OS in the complete cohort was 28.1 months (±3.4 months). Mean follow-up time was 47.1 months (±5.0 months). Two-year OS was 14.5%, 60.8% and 65.8% for patients treated within 21 days, 22–33 days after surgery and ≥34 days after surgery, respectively ().
Patients treated within 21 days from surgery had a significantly reduced OS compared to both groups with a longer interval between RT and surgery (p < .01). There was no significant difference between patients treated ≥34 days after surgery and patients treated 21–33 days from surgery (p = .210).
When excluding deaths occurring within 2 months from surgery, the benefit compared to patients starting within 21 days prevailed (p < .01). There was no significant difference between patients that started treatment ≥34 days after surgery and those that started treatment 22–33 days after surgery (p = .646).
In a multivariate model, significant predictors for OS were the timespan from surgery to start of RT (p < .01) year of RT (p < .01), histology (p < .01) and KPS before RT (p = .047). Age (p = .130), RPA (p = .101) and number of metastases (p = .829) had no significant impact on OS. Patients that began RT treatment within 21 days after surgery had a significantly worse OS than patients that began treatment 22–33 days (p = .017) and ≥34 days (p < .01) after surgery. There was no significant difference between patients treated ≥34 days after surgery compared to patients that started treatment 22–33 days from surgery (p = .301). This effect did not change after excluding patients that died within two months from surgery.
Local control
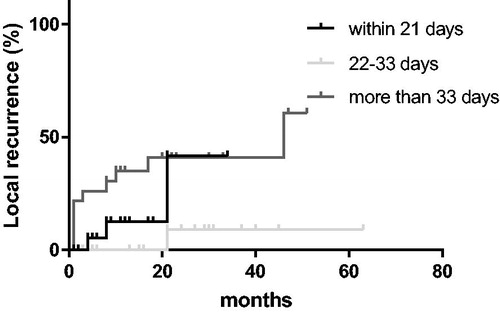
The 2-year local control rate in the complete cohort was 68.7%. Patients treated within 21 days, 22–33 days and ≥34 days after surgery had 2-year local control rates of 55.8%, 82.6% and 56.6%, respectively (). In the univariate analysis, a treatment start 22–33 days from surgery was significantly superior to a start ≥34 days from surgery (p = .049), not compared to an earlier start (p = .391). There was no significant difference between patients treated within 21 days and patients treated more than 33 days after surgery (p = .203). In a multivariate model, the only factor that approached significance on local control was the year RT was performed (p = .052). Histology (p = .767), KPS before RT (p = .431), RPA class (p = .900) and number of cerebral metastases (p = .807) were not significantly correlated with local control.
Locoregional control
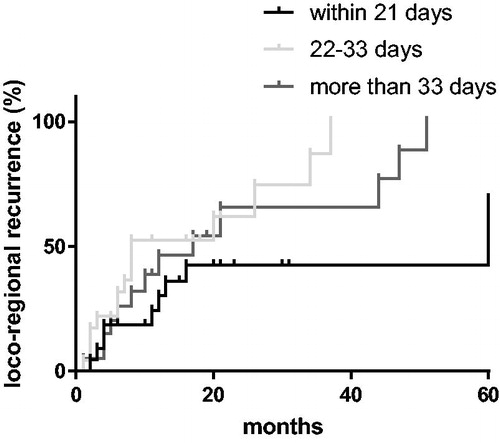
The 2-year locoregional control was 34.4%. There was no significant difference in locoregional control depending on RT timing (p = .508) (). Patients with more than one metastasis experienced a higher locoregional failure rate (p = .048). Histology (p = .572), year of RT (p = .567), RPA class (p = .572) and KPS before RT (p = .633) were not significantly associated with locoregional recurrence.
Discussion
We analyzed the outcome of 66 patients that received hypofractionated stereotactic radiotherapy to the resection cavity depending on the interval between surgery and the start of radiotherapy. Our study showed an improvement in OS for patients that started RT with an interval of more than 21 days after surgery compared to patients starting RT within 21 days from surgery. In an univariate model, local control was improved for patients beginning RT 22–33 days from surgery compared to a later start. In the multivariate analysis, this benefit did not prevail. The numbers of patients experiencing a local recurrence before RT begins, on the other hand, rises with longer intervals between surgery and radiotherapy. Locoregional control was not affected by the timing of RT.
The adjuvant radiation therapy to the resection cavity is a treatment that has constantly been advancing over the last decade. Adjuvant WBRT has been the standard of care for many years. As developments in systemic therapy for most entities have improved patients survival, increasing attention has been drawn to cognitive impairments due to whole brain radiotherapy. Local radiotherapeutic options (i.e., stereotactic radiosurgery or hypofractionated RT) with reduced neurologic impairment and comparable survival have evolved [Citation4,Citation5]. To our knowledge, this is the first study analyzing the optimal timing of adjuvant RT to the resection cavity of brain metastases.
The timing of RT has long been recognized as a factor influencing treatment outcome. Multiple studies have examined the role of RT timing in head and neck cancer, the majority of which have demonstrated a negative prognostic impact of long intervals between surgery and RT [Citation11–13]. For breast cancer patients, an increase in loco-regional recurrences has been demonstrated for a delayed adjuvant treatment beyond 6 weeks from surgery, particularly for patients with positive resection margins [Citation14,Citation15]. In the light of this knowledge, one would expect a short interval between surgery and RT to be beneficial in the adjuvant treatment of brain metastases as they are rarely removed with large safety margins. However, the previously described evidence focused on local or regional disease, and the underlying studies excluded patients with any systemic disease. The local environment on metastatic sites, in our case the surrounding brain tissue, might influence the rate of local recurrences. In glioblastoma, two studies have revealed an inferior outcome for patients treated within a period of 24 days and 4 weeks after surgery, respectively [Citation9,Citation10]. Other studies could not find an impact of the timing of RT treatment in glioblastoma or high-grade childhood glioma [Citation7,Citation8]. We found that early radiation within 21 days significantly decreased OS compared to patients that started radiation therapy 22–33 days or ≥34 days after surgery for brain metastases. Preclinical findings in rats, irradiated 11 and 21 days after hemisection of the frontal lobe suggested that early irradiation after brain surgery may result in augmented tissue damage [Citation16]. Certainly, findings from animal models cannot be adopted unaltered into humans. Nevertheless, higher rates of tissue damage could be a possible explanation for decreased OS rates in patients treated early after surgery. Another explanation for the lower OS rates in patients that received radiotherapy within 21 days from surgery is a negative selection bias in this patient group as the Karnofsky performance score is significantly lower compared to the other groups. It is imaginable that patients with a reduced general health status after surgery are more likely to be scheduled for radiotherapy within a short interval after surgery since discharge from the hospital is not possible. Furthermore, RPA class, which amongst other factors takes systemic disease status into account, favored the groups with longer interval between surgery and RT confirming the assumption that confounding factors are at least partly responsible for the OS benefit perceived [Citation17]. These confounders, however, were adjusted for in the multivariate analysis which resulted in similar results showing an improved OS for patients starting radiotherapy ≥21 days from surgery. As no information on reasons for certain intervals between surgery and RT could be retrieved in the majority of cases, it remains unclear whether RT scheduling was influenced by factors distorting the results of this study. Even though not statistically significant, we observed the highest local control rates for patients treated in the time frame of 22–33 days after surgery. In the univariate model, we even perceived a significant reduction in local control for longer intervals suggesting that RT should not be postponed much longer. Furthermore, local recurrence before RT was correlated with the time interval between surgery and RT. This is not surprising as historic data demonstrated 2-year local recurrence rates of approximately 60% after surgical resection of brain metastasis [Citation3]. A possible explanation for similar local recurrence rates in the groups with the earliest and longest interval between surgery and RT despite of a greater recurrence risk before RT is the fact that target volume definition shortly after surgery is more complicated and volumetric changes to the resection cavity occur more frequently [Citation18,Citation19].
In addition, local recurrence before RT did not influence overall local control after RT was performed, suggesting an ablative effect of the performed radiotherapy. The effect of tumor size and prescription dose on local control in radiosurgery of brain metastases, on the other hand, is well known [Citation20]. In this study, the dose applied to the resection cavity equals a biological effective dose (BED) of approximately 52–96 Gy (depending on the alpha/beta of the treated tumor). The typical dose used for the treatment of WBRT after surgical resection of brain metastasis is 30–36 Gy in fractions of 3 Gy which corresponds to a BED of 39–72 Gy. These significantly higher doses used in stereotactic radiotherapy may have diminished the effect of macroscopic tumor within the resection cavity on local control to a certain extent [Citation21]. Nevertheless, residual or recurrent tumor should be avoided as both local failure and the risk of radionecrosis increases with size. This is of particular relevance in the case of resected metastases due to the greater sizes of resection cavities compared to the initial metastases and the resulting limitations to the prescription dose [Citation18]. Hypoxic conditions shortly after surgery might also be a reason rendering the remaining tumor cells less susceptible to radiotherapy [Citation22]. The only other factor that significantly affected OS and local recurrence was the year RT was performed. This is due to advances in surgery, systemic therapies and RT. In the last few years, supra-marginal resection has become the standard of care in brain metastasis surgery aided by refined preoperative mapping and intraoperative monitoring techniques [Citation23,Citation24]. The introduction of new targeted therapies and check-point inhibitors into clinical practice, for example, prolonged OS for many entities.
There are certain limitations to this study; predominantly owing to its retrospective nature. First of all, the OS was calculated from the date of surgery for a list of patients that was derived from a database of the department of radiation oncology. Therefore, the patients in the group treated 22–33 days and more than 33 days from surgery will automatically lack patients that died earlier after surgery. We intended to overcome this bias by performing a second analysis excluding patients that died within 2 months from surgery. Since this analysis showed similar results, we assumed that the perceived effect was independent of this bias.
Secondly, as the timing of RT start was not randomized certain patient conditions could have prompted the treating physician to select an earlier or delayed start of RT. Imaginable reasons for a very short interval between surgery and RT are, for instance, a reduced KPS after surgery impeding a discharge from the hospital or an aggressive tumor biology. On the other hand, patients with a bad performance score or surgical complications might be transferred to a rehabilitation program first and irradiated with a delayed timing. Furthermore, known predictors for OS and local control were not actively balanced between the groups. In order to minimize these confounders a multivariate analysis was performed. This indisputably does not account for all the possible potentially unknown confounding parameters.
Conclusion
A short delay in the start of radiotherapy does not seem to negatively impact the outcome in patients with resected brain metastases. We even observed an unexpected reduction in OS in patients treated within 21 days from surgery. This may, however, be the result of a negative selection bias in this group. The risk for local tumor recurrence before RT rises with longer interval between surgery and radiotherapy leading to macroscopic tumor in the resection cavity at the time of radiotherapy. However, a short delay of RT after surgery did not significantly increase the rate of local recurrences compared with a start within 21 days of surgery. Consequently, timely initiation of RT is indicated to prevent early recurrences while balancing time for recovery and wound healing are essential. Further studies are needed to further narrow down the optimal timing of postoperative radiotherapy to the resection cavity.
Acknowledgments
The authors thank our team of technicians for excellent patient care.
Disclosure statement
Sophia Scharl received a travel grant from Mundipharma GmbH and NovoCure Ltd.; Christoph Straube received a scholarship from Medac GmbH, received a travel grant from NovoCure Ltd.; Bernhard Meyer work as consultants for BrainLab and Nexstim; Claus Zimmer has served on scientific advisory boards for Philips and Bayer Schering; serves as co-editor on the Advisory Board of Clinical Neuroradiology; has received speaker honoraria from Bayer-Schering and Philips and has received research support and investigator fees for clinical studies from Biogen Idec, Quintiles, MSD Sharp & Dome, Boehringer Ingelheim, Inventive Health Clinical UK Ltd., Advance Cor, Brainsgate, Pfizer, Bayer-Schering, Novartis, Roche, Servier, Penumbra, WCT GmbH, Syngis, SSS International Clinical Research, PPD Germany GmbH, Worldwide Clinical Trials Ltd., Phenox, Covidien, Actelion, Medivation, Medtronic, Harrison Clinical Research, Concentric, Penumbra, Pharmtrace, Reverse Medical Corp., Premier Research Germany Ltd., Surpass Medical Ltd. and GlaxoSmithKline; Stephanie E. Combs has served on Advisory Board of Bristol-Myers-Squibb (BMS); Advisory board and Speaker’s Bureau for BrainLab; Advisory Board of Roche, Daiichi Sankyo and Varian Medical Systems. Has received Speakers Honoraria from BrainLab, Tomotherapy, Dr. Sennewald, BMS, Varian Medical Systems, Elekta, Novocure and Medac GmbH. The remaining authors declare no competing financial interest.
References
- Sul J, Posner JB. Brain metastases: epidemiology and pathophysiology. Cancer Treat Res. 2007;136:1–21.
- Kocher M, Wittig A, Piroth MD, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014;190:521–532.
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489.
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060.
- Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040.
- Steinmann D, Maertens B, Janssen S, et al. Hypofractionated stereotactic radiotherapy (hfSRT) after tumour resection of a single brain metastasis: report of a single-centre individualized treatment approach. J Cancer Res Clin Oncol. 2012;138:1523–1529.
- Azizi AA, Paur S, Kaider A, et al. Beeinflusst die Zeitspanne zwischen Tumorresektion und Strahlentherapie das Überleben bei Kindern mit hochgradigen Gliomen? Strahlenther Onkol. 2018;194:552–559.
- Seidlitz A, Siepmann T, Löck S, et al. Impact of waiting time after surgery and overall time of postoperative radiochemotherapy on treatment outcome in glioblastoma multiforme. Radiat Oncol. 2015;10:172.
- Adeberg S, Bostel T, Harrabi S, et al. Impact of delays in initiating postoperative chemoradiation while determining the MGMT promoter-methylation statuses of patients with primary glioblastoma. BMC Cancer. 2015;15:558.
- Blumenthal DT, Won M, Mehta MP, et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27:733–739.
- Tam M, Wu SP, Gerber NK, et al. The impact of adjuvant chemoradiotherapy timing on survival of head and neck cancers. Laryngoscope. 2018;128:2326–2332.
- Tribius S, Donner J, Pazdyka H, et al. Survival and overall treatment time after postoperative radio(chemo)therapy in patients with head and neck cancer. Head Neck. 2016;38:1058–1065.
- Fujiwara RJT, Judson BL, Yarbrough WG, et al. Treatment delays in oral cavity squamous cell carcinoma and association with survival. Head Neck. 2017;39:639–646.
- Kim K, Chie EK, Han W, et al. Impact of delayed radiotherapy on local control in node-negative breast cancer patients treated with breast-conserving surgery and adjuvant radiotherapy without chemotherapy. Tumori. 2011;97:341–344.
- Vujovic O, Cherian A, Yu E, et al. The effect of timing of radiotherapy after breast-conserving surgery in patients with positive or close resection margins, young age, and node-negative disease, with long term follow-up. Int J Radiat Oncol Biol Phys. 2006;66:687–690.
- Peker S, Abacioglu U, Sun I, et al. Irradiation after surgically induced brain injury in the rat: timing in relation to severity of radiation damage. J Neurooncol. 2004;70:17–21.
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751.
- Scharl S, Kirstein A, Kessel KA, et al. Volumenveränderungen der Resektionshöhlen nach Operation von Hirnmetastasen – Konsequenzen für die stereotaktische Strahlentherapie. Strahlenther Onkol. 2019;195:207–217.
- Atalar B, Choi CYH, Harsh GR, et al. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery. 2013;72:180–185; discussion 185.
- Moraes FY, Winter J, Atenafu EG, et al. Outcomes following SRS for small- to medium-sized brain metastases are exceptionally dependent upon tumor size and prescribed dose. Neuro-Oncology. 2018;21:242–251.
- Wiggenraad R, Verbeek-de Kanter A, Kal HB, et al. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol. 2011;98:292–297.
- Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57(Suppl 1):i90–i98.
- Krieg SM, Picht T, Sollmann N, et al. Resection of motor eloquent metastases aided by preoperative nTMS-based motor maps-comparison of two observational cohorts. Front Oncol. 2016;6:261.
- Krieg SM, Schäffner M, Shiban E, et al. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: clinical article. J Neurosurg. 2013;118:1269–1278.