Abstract
Background: Preoperative genetic testing affects the surgical decision-making among women with breast cancer. To avoid breast-conserving surgery and to offer the possibility of mastectomy with immediate reconstruction in high-risk patients, genetic testing for pathogenic variants in BRCA1 or BRCA2 and a pedigree-based familial breast cancer risk assessment was offered to younger women with breast cancer in Denmark. We evaluated the impact of the risk stratification through genetic counseling on the uptake of contralateral prophylactic mastectomy (CPM).
Material and methods: The prospective cohort study included all women with unilateral breast cancer before the age of 45 who participated in a genetic counseling program during their primary diagnostics in the Central Denmark Region (2013–2018). Each patient was followed from the time of the genetic test result to the end of follow-up to estimate the long-term uptake of CPM as a competing risk-adjusted cumulative incidence. We compared the uptake of CPM between the various genetic risk categories, ages of onset, and family histories in a multivariable Cox proportional hazards regression model, reporting hazard ratios (HR) with two-sided 95% confidence intervals (CIs).
Results: 156 females, aged 21–44, learned their genetic test result within a median of 92 days [interquartile range (IQR): 75–114]. The maximal follow-up was 3.8 years (median 1.8; IQR: 0.49–2.5), after which 33% (95% CI: 24–42%) of the patients had undergone CPM. The uptake of CPM was inversely associated with the age of onset (HR 0.92; 95% CI: 0.86–0.98) and significantly higher among BRCA carriers (HR 2.9; 95% CI: 1.3–6.8) and patients from the high risk of breast cancer families (HR 5.6; 95% CI: 1.9–16) compared to the lower genetic risk categories.
Conclusion: The risk stratification obtained through genetic counseling had a considerable impact on the surgical decision-making among younger women with breast cancer at long-term follow-up.
Introduction
Contralateral prophylactic mastectomy (CPM) after unilateral breast cancer has seen a steady rise worldwide with the highest increase in the younger age groups [Citation1–Citation4]. Although the trend has been particularly well-documented in North America, less is known for Europe during the same period [Citation5,Citation6].
The uptake of CPM is affected by multiple factors such as age, family history, genetic test results, personality traits, and the surgical options and recommendations [Citation7–Citation9]. CPM reduces the risk of contralateral breast cancer by about 95%, whereas its impact on long-term survival is unsettled [Citation10]. Carriers of pathogenic variants in BRCA1 or BRCA2 demonstrate improved long-term survival after CPM [Citation11]. For non-BRCA carriers the greatest survival benefit is expected to be in those with a good breast cancer prognosis, a low mortality risk of other causes, and a high risk of contralateral breast cancer [Citation12]. Younger women with a strong family history of breast cancer who test negative for pathogenic variants in BRCA1 or BRCA2 still face a considerable risk of contralateral breast cancer [Citation13].
Preoperative genetic testing for pathogenic variants in BRCA1 or BRCA2 is known to affect surgical decision-making among newly diagnosed breast cancer patients [Citation14]. To identify BRCA carriers prior to their definitive surgery, genetic counseling was introduced into the primary diagnostic work-up for eligible women in the Central Denmark Region in 2013. The aim was to avoid breast-conserving surgery in high-risk patients and in case of high risk to offer the possibility of mastectomy with immediate reconstruction. To be eligible, the woman should have a personal and/or family history meeting the national guideline criteria for genetic counseling such as breast cancer before 40 years of age, pre-menopausal triple negative or bilateral breast cancer, or a family history suggestive of hereditary breast and ovarian cancer. The genetic counseling comprised genetic testing for pathogenic variants in BRCA1 or BRCA2 and a pedigree-based familial risk assessment.
International guidelines agree that patients should be counseled about their future contralateral breast cancer risk, but there are no general recommendations about CPM for non-BRCA carriers [Citation12,Citation15]. We identified a need to evaluate how the risk stratification obtained through genetic counseling affected the long-term uptake of CPM among younger women with breast cancer.
Material and methods
Study population
Women were recruited from the Department of Clinical Genetics, Aarhus University Hospital, in the period 1 January 2013 to 31 May 2018. The department has the entire Central Denmark Region as catchment area (approximately 1.3 million inhabitants).
We included women referred to genetic counseling during their primary diagnostic workup for invasive breast cancer and excluded women ≥45 years of age or with a personal history of breast cancer, ovarian cancer, or BRCA testing prior to the inclusion period. We also excluded women with bilateral breast cancer, which we defined as contralateral breast cancer within three months of the diagnosis.
Genetic risk stratification
The genetic counseling was led by a clinical geneticist. There were multiple clinical geneticists and genetic counselors working under supervision. During pretest counseling, genetic testing was discussed with the patient. A blood sample from each woman was tested for pathogenic variants in BRCA1 or BRCA2 by next generation sequencing. A family history was obtained and cancer diagnoses were verified in the medical records after written consent. During post-test counseling, the patient was informed about her genetic test result and the result of her genetic risk stratification.
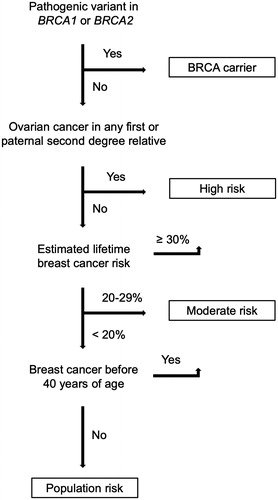
Women found to carry a pathogenic variant in BRCA1 or BRCA2 (BRCA carrier) were counseled as having an elevated risk of breast cancer. Non-BRCA carriers were counseled according to three familial breast cancer risk categories defined by the Danish Breast Cancer Cooperative Group: high risk, moderate risk, and population risk. According to the national guidelines, patients were perceived to have a high risk if they belonged to a family in which breast cancer and ovarian cancer had occurred in the same patient, first degree relatives, or second degree relatives through a male. Also the rare case of a male breast cancer history would indicate a familial high risk. If a family member, such as the patient herself, had been diagnosed with breast cancer before 40 years of age, the patient was assessed as having at least familial moderate risk. For other patients, the familial risk was assessed on the basis of the calculated lifetime risk for an unaffected relative to the patient using the BOADICEA model [Citation16]. A simplified flow diagram illustrates the genetic risk stratification (). The national guidelines stated that risk-reducing surgery could be discussed with a woman who was either a BRCA carrier or had a familial high risk of breast cancer. In clinical practice, such patients were entitled to opt to undergo CPM; however, the treatment was not specifically recommended.
Data analysis
To record cancers and surgical procedures, pathology reports with dates and diagnoses were obtained from the Danish national pathology database, linked through a 10-digit personal identification number assigned to all Danish residents [Citation17].
The long-term uptake of CPM was examined in a survival model. We were able to follow the patients from the date of diagnosis; however, entry was delayed until the date the patient received the genetic test result or three months after the breast cancer diagnosis, whichever came latest. The purpose of the delayed entry was to avoid conditioning on the future as the genetic risk category and bilateral breast cancer status could first be determined at that time. The follow-up ended on the date of CPM, contralateral breast cancer, death, or last follow-up, whichever came first. Kaplan–Meier plots with number at risk tables were included for visualization. The uptake of CPM was estimated as the cumulative incidence in the presence of contralateral breast cancer and death as competing risks [Citation18].
Hazard ratios (HRs) were estimated for potential predictors of CPM in a Cox proportional hazards regression model. We considered the following exposure variables as potential predictors: genetic risk category, age of onset for breast cancer, family history of breast cancer, or family history of ovarian cancer. Family history was defined as having an affected first or second degree relative. Age of onset for breast cancer was included in the regression model as a continuous variable. Each exposure variable was evaluated alone (univariable model) and then together with all other exposure variables (multivariable model). The proportional hazards assumption was verified for each exposure variable category on the basis of log-log plots and scaled Schoenfield residuals.
p values were obtained with the Fisher’s exact test for categorical variables, whereas for age of onset the non-parametric Wilcoxon rank-sum and Kruskal–Wallis tests were used, as appropriate.
Statistical significance and confidence intervals (CI) were reported at the two-sided 95% level. All data were analyzed in Stata 12.1 (StataCorp, College Station, TX).
The study was registered with the Danish Data Protection Agency (1-16-02-102-17). Approval was granted from the Danish Patient Safety Authority (3-3013-2912/1) to obtain data from medical records without the need for consent. Under Danish law, the study was not reportable to an ethics committee.
Results
The study cohort included 156 women undergoing genetic testing and counseling due to early-onset breast cancer (age range: 21–44). The patients were treated for their cancer by either breast-conserving surgery and adjuvant therapy or neoadjuvant therapy. We had excluded patients for the following reasons: bilateral breast cancer (n = 6), less than three months of follow-up after the breast cancer diagnosis (n = 5), undergoing CPM before receiving the genetic test result (n = 3), not having returned to receive the genetic test result (n = 2), or carrying variants of uncertain significance in BRCA1 or BRCA2 (n = 1).
The result of the genetic testing was scheduled before radiation therapy or final surgery. This meant that the women learned their genetic test result within a median of 92 days [interquartile range (IQR): 75 to 114] from the breast cancer diagnosis. The patients received a concluding risk assessment, which stratified them into one of four genetic risk categories: BRCA carriers (n = 17), familial high risk (n = 15), familial moderate risk (n = 113), or population risk (n = 11). As expected, the patients in the four genetic risk categories differed by age of onset and family history ().
Table 1. Patient characteristics.
Despite the differences between the genetic risk categories, the age of onset for breast cancer was not associated with having a family history of breast cancer (Wilcoxon rank-sum, p = .10) or ovarian cancer (Wilcoxon rank-sum, p = .99). Also there was no significant association between having a family history of breast cancer and ovarian cancer (Fisher’s exact, p = .10).
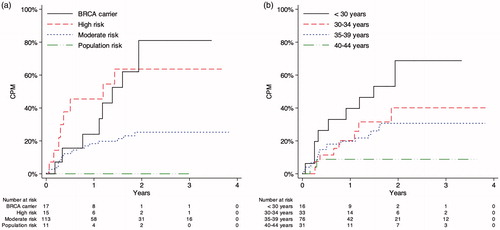
We were able to follow the patients for a median observation period of 1.8 years (IQR: 0.49 to 2.5 years) from the start of follow-up (). Within a year, 20% (95% CI: 14% to 28%) of the patients had opted to undergo CPM. After the maximal follow-up period of 3.8 years, the uptake of CPM had grown to 33% (95% CI: 24% to 42%). Metachronous contralateral cancer or death occurred as the first outcomes in the competing risk analysis at cumulative incidences of, respectively, 0.83% (95% CI: 0.075% to 4.1%) and 10% (95% CI: 4.8% to 18%).
Table 2. Follow-up data.
At the end of follow-up, BRCA carriers had the highest uptake of CPM, 72% (95% CI: 36% to 90%), followed by women in the familial high risk, 64% (95% CI: 31% to 84%), and familial moderate risk categories, 25% (95% CI: 16% to 34%). No patients in the population risk category underwent CPM (). The uptake of CPM also varied considerably with age, decreasing from 65% (95% CI: 32% to 85%) in the youngest to 8.8% (95% CI: 1.5% to 25%) in the oldest age group ().
Since no patients in the population risk category underwent CPM, we combined the two lowest genetic risk categories in the Cox regression analysis. The uptake of CPM was significantly associated with the genetic risk category and age of onset for breast cancer, but not with having a family history of breast cancer or ovarian cancer ().
Table 3. Evaluated predictors of CPM in the Cox regression model.
Of the three patients who underwent CPM before receiving their genetic test result, there was one patient in each of the moderate risk, familial high risk, and BRCA carrier categories, and the age range was 33 to 40 years. Only the patient in the familial high risk category had a family history of breast cancer, whereas none of the patients had a family history of ovarian cancer. Thus, we found no obvious reason why the three patients underwent CPM before receiving their genetic test result.
Discussion
We evaluated the uptake of CPM among younger women with breast cancer who participated in a genetic counseling program during their primary diagnostics of breast cancer. Around three months lasted from the breast cancer diagnosis to the day the woman learned her genetic test result. Although the process might have been speeded up through genetic testing at the primary diagnostic unit, we prioritized testing in a setting of genetic counseling and demonstrate this to be feasible within just three months. A further gain, was the increased number of risk categories, as the pedigree-based risk assessment relied not only on the BRCA status but also on the family history of the woman.
The uptake of CPM among younger women with breast cancer in Denmark was comparable to the uptake among age-matched women in the United States [Citation1]. Most BRCA carriers and women with a familial high risk of breast cancer underwent CPM, whereas CPM was less common among women in the familial moderate risk and population risk categories, consistent with a high uptake of bilateral prophylactic mastectomy among young BRCA carriers with no personal history of breast cancer [Citation19]. Age and family history were previously reported to affect the surgical decision-making among breast cancer patients [Citation14,Citation20]. After adjusting for these potential confounders in the multivariable regression model, the genetic risk stratification was still associated with a strong impact on the uptake of CPM.
CPM reduces the risk of contralateral breast cancer and cancer-related anxiety [Citation10]. Well-established risk factors for contralateral breast cancer include early-onset primary breast cancer, a pathogenic variant in a high-risk gene, family history, and non-genetic factors [Citation12]. A registry-based study from Denmark found a 5.5% 10-year risk of contralateral breast cancer among women under 45 years of age [Citation21], whereas the individual risk to a woman can be further differentiated on the basis of age of onset, family history, and BRCA status [Citation13]. The evidence of long-term survival benefit after CPM is limited for genetically disposed individuals [Citation10], and the recommendations therefore vary between international guidelines [Citation12,Citation15]. The surgeon's recommendation affects the final decision-making, as women without hereditary risk are more likely to undergo CPM if the surgeon neither recommends for or against CPM than if the surgeon recommends against CPM [Citation9,Citation22].
A proximate family history of breast cancer and ovarian cancer was enough to classify a family as high-risk despite negative BRCA testing. As a consequence, more than half of the women counseled as having a familial high risk had a first or second degree relative with ovarian cancer compared to only a small minority of patients in the familial moderate risk and population risk categories. Since a family history of ovarian cancer is not itself a known risk factor for contralateral breast cancer, this practice may have led to unnecessary anxiety among some of the patients. The national guidelines are currently being updated to improve the precision of breast cancer risk assessment through systematic use of the BOADICEA model. Inclusion of moderate risk genes, polygenic risk scores, and environmental factors may improve the precision of genetic risk assessment in the future [Citation23].
Due to the moderate size of the study population, we had to combine the two lowest genetic risk categories in the Cox proportional hazards regression model. This was reasonable, given that CPM was considered an option for high-risk patients, not population risk or moderate risk individuals. Yet it would have been interesting to compare the uptake between these two genetic risk categories as well. As the genetic counseling program was aimed at identifying women at high risk of breast cancer, it was offered exclusively to those patients more likely to harbor pathogenic variants in BRCA1 or BRCA2, such as younger women or those with triple negative breast cancer or a positive family history. The absence of a control group, that did not undergo genetic counseling, limited us from assessing the overall impact of the program. As the patients were not asked to answer decision-making questionnaires, we were restricted to observe the long-term differences in CPM uptake between groups defined by genetic risk category, age of onset, and family history, whereas the impact of additional motivation factors did not come to light. As the genetic testing was restricted to BRCA1 and BRCA2, other studies will be needed to assess the impact of positive test results for moderate risk genes, such as PALB2, CHEK2, and ATM. Interestingly, a CPM uptake comparable to BRCA carriers was recently reported for non-BRCA carriers with positive multigene panel testing results [Citation24].
Given the steady increase in CPM up to 2 years after the breast cancer diagnosis, a strength of the study was the analysis of long-term follow-up data, providing clinically important data on the long-term impact of risk stratification through genetic counseling. Consistent with the aim of the program, we found very few high-risk patients not opting for bilateral mastectomy with immediate reconstruction.
Conclusion
The risk stratification obtained through genetic counseling had a considerable impact on the surgical decision-making among younger women with breast cancer at long-term follow-up.
| Abbreviations | ||
| BOADICEA | = | the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm |
| BRCA carrier | = | pathogenic variant in BRCA1 or BRCA2 |
| CI | = | confidence interval |
| CPM | = | contralateral prophylactic mastectomy |
| HR | = | hazard ratio |
| IQR | = | interquartile range |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wong SM, Freedman RA, Sagara Y, et al. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg. 2017;265:581–589.
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. JCO. 2007;25:5203–5209.
- Kurian AW, Lichtensztajn DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA. 2014;312:902–914.
- Kiely BE, Jenkins MA, McKinley JM, et al. Contralateral risk-reducing mastectomy in BRCA1 and BRCA2 mutation carriers and other high-risk women in the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab). Breast Cancer Res Treat. 2010;120:715–723.
- Neuburger J, Macneill F, Jeevan R, et al. Trends in the use of bilateral mastectomy in England from 2002 to 2011: retrospective analysis of hospital episode statistics. BMJ Open. 2013;3:e003179.
- Guth U, Myrick ME, Viehl CT, et al. Increasing rates of contralateral prophylactic mastectomy - a trend made in USA? Eur J Surg Oncol. 2012;38:296–301.
- Wang F, Amara D, Peled AW, et al. Negative genetic testing does not deter contralateral prophylactic mastectomy in younger patients with greater family histories of breast cancer. Ann Surg Oncol. 2015;22:3338–3345.
- van Driel CMG, Oosterwijk JC, Meijers-Heijboer EJ, et al. Psychological factors associated with the intention to choose for risk-reducing mastectomy in family cancer clinic attendees. Breast. 2016;30:66–72.
- Jagsi R, Hawley ST, Griffith KA, et al. Contralateral prophylactic mastectomy decisions in a population-based sample of patients with early-stage breast cancer. JAMA Surg. 2017;152:274–282.
- Carbine NE, Lostumbo L, Wallace J, et al. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev. 2018;4:CD002748.
- Metcalfe K, Gershman S, Ghadirian P, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ. 2014;348:g226.
- Hunt KK, Euhus DM, Boughey JC, et al. Society of surgical oncology breast disease working group statement on prophylactic (risk-reducing) mastectomy. Ann Surg Oncol. 2017;24:375–397.
- Reiner AS, John EM, Brooks JD, et al. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women's Environmental Cancer and Radiation Epidemiology Study. JCO. 2013;31:433–439.
- Schwartz MD, Lerman C, Brogan B, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. JCO. 2004;22:1823–1829.
- Wright FC, Look Hong NJ, Quan ML, et al. Indications for contralateral prophylactic mastectomy: a consensus statement using modified delphi methodology. Ann Surg. 2018;267:271–279.
- Lee AJ, Cunningham AP, Kuchenbaecker KB, Consortium of Investigators of Modifiers of B, Breast Cancer Association C, et al. BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer. 2014;110:535–545.
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25.
- Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103.
- Skytte AB, Gerdes AM, Andersen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: uptake and timing. Clin Genet. 2010;77:342–349.
- Uyei A, Peterson SK, Erlichman J, et al. Association between clinical characteristics and risk-reduction interventions in women who underwent BRCA1 and BRCA2 testing: a single-institution study. Cancer. 2006;107:2745–2751.
- Rasmussen CB, Kjaer SK, Ejlertsen B, et al. Incidence of metachronous contralateral breast cancer in Denmark 1978-2009. Int J Epidemiol. 2014;43:1855–1864.
- Katz SJ, Janz NK, Abrahamse P, et al. Patient reactions to surgeon recommendations about contralateral prophylactic mastectomy for treatment of breast cancer. JAMA Surg. 2017;152:658.
- Lee A, Mavaddat N, Wilcox AN, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019.
- Elsayegh N, Webster RD, Gutierrez Barrera AM, et al. Contralateral prophylactic mastectomy rate and predictive factors among patients with breast cancer who underwent multigene panel testing for hereditary cancer. Cancer Med. 2018;7:2718–2726.