Abstract
Background: The relation between cardiovascular disease (CVD) present at the time of cancer diagnosis and Health-Related Quality of Life (HRQoL) assessed years after cancer diagnosis has – to our knowledge – not been studied. The objective is, therefore, to examine the relation between co-morbid CVD at cancer diagnosis and HRQoL among cancer survivors diagnosed with colorectal, thyroid, prostate, endometrium, ovarian cancer, melanoma, (non-)Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), or multiple myeloma (MM) in an exploratory population-based cross-sectional study.
Material and methods: Analyses were performed on combined data sets from the PROFILES and Netherlands Cancer Registry (NCR). Data on co-morbid CVD at cancer diagnosis was extracted from the NCR. HRQoL was measured via PROFILES at a median of 4.6 years after cancer diagnosis. General Linear Model Analyses were run for the total group of cancer survivors and for each malignancy.
Results: In total, 5930 cancer survivors (2281 colorectal, 280 thyroid, 1054 prostate, 177 endometrium, 389 ovarian cancer, 212 melanoma, 874 non-Hodgkin and 194 Hodgkin lymphoma, 242 CLL, and 227 MM survivors) were included. For the total group, survivors who had a CVD at cancer diagnosis (n = 1441, 23.4%) reported statistically significant and clinically important lower scores on global QoL and physical functioning and higher scores for dyspnea (p < .05) compared to those without CVD. Co-morbid CVD at cancer diagnosis was negatively related to global QoL, the five functional scales and the symptoms fatigue and dyspnea across most malignancies (i.e., colorectal, and prostate cancer, non-Hodgkin lymphoma, ovarium cancer, melanoma, and CLL). No significant relations were found among thyroid and endometrium cancer, Hodgkin lymphoma and MM survivors, likely due to small numbers.
Conclusion: In conclusion, co-morbid CVD at cancer diagnosis was negatively related to HRQoL, especially to global QoL, physical and role functioning, and the symptoms fatigue and dyspnea.
Introduction
Cancer and cardiovascular disease (CVD) are two common causes of morbidity and mortality worldwide. It is estimated that 20–30% of patients with cancer already have a co-morbid CVD at the time of their cancer diagnosis [Citation1]. Having a preexisting CVD often affects cancer treatment options, frequently resulting in less aggressive treatment [Citation2]. Alternatively, CVD’s are well-known late effects of cancer treatment [Citation3,Citation4]. There is increasing attention for the co-occurrence of CVD and cancer. The International CardiOncology Society advocates that both clinical practice and research should re-focus from a disease-specific approach to a whole person approach – taking into account information on both cancer and CVD [Citation5]. This approach is necessary to reduce the impact of CVD among cancer survivors.
Within the field of oncology, there has been increasing awareness for long-term outcomes including patient-reported outcomes (PROs) such as Health-Related Quality of Life (HRQoL). The presence of CVD in cancer survivors has been negatively associated with patients’ clinical health outcomes, such as survival [Citation6]. Additionally, having one or more co-morbid conditions at the time of HRQoL assessment among cancer survivors has a negative impact on HRQoL [Citation7,Citation8]. The relation between CVD present at the time of cancer diagnosis and HRQoL assessed years after cancer diagnosis has – to our knowledge – not been studied. The objective of this study is, therefore, to examine the relation between co-morbid CVD at cancer diagnosis and HRQoL among long-term cancer survivors diagnosed with colorectal, thyroid, prostate, endometrium, or ovarian cancer, melanoma, (non-)Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), or multiple myeloma (MM). Data were analyzed for the total group of cancer survivors and for each malignancy separately. We hypothesize that those cancer survivors who had a co-morbid CVD at cancer diagnosis experienced a poorer HRQoL. Additionally, we examined whether the relation between co-morbid CVD and HRQoL differed by gender, age, time since diagnosis, systemic therapy, radiation or hormone treatment.
Material and methods
Study design and setting
This study uses data from the PROFILES (‘Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship’) and Netherlands Cancer Registry (NCR) [Citation9]. The PROFILES registry is an ongoing data collection of PROs within the sampling frame of the NCR and can be linked with clinical data of all individuals newly diagnosed with cancer in the Netherlands[Citation9].
Study population
The current study combined several cohorts from the PROFILES registry; i.e., survivors of colorectal, thyroid, prostate, endometrium, and ovarian cancer, melanoma, (non-)Hodgkin lymphoma, CLL, or MM as primary cancer [Citation10]. Survivors were included during 2008–2014. Eligible participants were ≥18 years at cancer diagnosis and excluded if they were not able to complete the questionnaire according to their (ex-)attending specialist (i.e., due to severe cognitive impairments, being too ill or those who were not sufficiently flued in Dutch). Ethical approval was obtained for all study samples separately, from local Dutch certified medical ethics committees.
Data collection
Description of the data collection has been described previously [Citation9]. In short, cancer survivors were informed about the study via a letter by their (ex-)attending specialist. This letter contained an informed consent form and a paper questionnaire or a secured link to a web-based informed consent form and an online questionnaire. Patients could return a postcard to request a paper-and-pencil questionnaire. For each participant informed consent was obtained.
Measures
Co-morbid CVD
Data on co-morbid CVD at cancer diagnosis has been extracted from medical records by trained registry personnel and was registered in the NCR. Co-morbidity data have been recorded since 1993 by screening previous admissions, letters of referral from and discharge to general practitioners, the medical history, current medication and preoperative assessments [Citation11]. Internal validation studies were performed evaluating the quality by randomly checking completeness and accuracy of the registry personnel extracting co-morbidity information from the medical records [Citation12]. The quality of this data is high because of thorough training of the registrars and computerized consistency checks at regional and national level. Completeness is estimated to be at least 95% [Citation12]. CVDs were classified into: myocardial infarction; angina pectoris; coronary artery disease; cardiac decompensation (heart failure); cardiomyopathy; valve problems; heart transplant; problems with heart rhythm; peripheral arterial disease; thrombosis; and cerebrovascular disease.
Health-Related quality of life (HRQoL)
HRQoL was measured by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) [Citation13]. This 30-item questionnaire comprises five functional scales: physical, role, cognitive, emotional and social functioning; a global quality of life scale; three symptom scales on fatigue, pain, and nausea/vomiting; and six items assessing dyspnea, insomnia, loss of appetite, constipation, diarrhea and financial impact. A higher score on the functional scales and global QoL means better functioning and HRQoL, whereas a higher score on the symptom scales indicates more complaints.
Socio-demographics and clinical characteristics
Age at diagnosis and gender were obtained from the NCR. Patients’ marital status and educational level were assessed via questionnaire.
The clinical characteristics of cancer stage and primary treatment were obtained from the NCR. Cancer stage was classified according to TNM [Citation14] or Ann Arbor Code for (non-)Hodgkin lymphoma. Primary treatment was classified into surgery, systemic therapy (chemotherapy, targeted therapy, and immune therapy), radiotherapy (including brachytherapy), and hormone treatment. Number of co-morbidities other than CVD at the time of survey was assessed with the adapted Self-administered Co-morbidity Questionnaire (SCQ) [Citation15] and categorized into 0 co-morbidity, 1 co-morbidity or ≥2 co-morbidities.
Statistical analyses
Socio-demographics and clinical characteristics of those with versus those without co-morbid CVD at cancer diagnosis were compared by means of chi-square or t-tests. We examined the relation between having a co-morbid CVD at cancer diagnosis and HRQoL by means of linear regression analyses using the general linear model (GLM). Analyses were run for the total group of cancer survivors and for each malignancy separately. We included global QoL and the five functional scales, together with the symptoms of fatigue and dyspnea, as these are known to be impacted by CVD. Gender, age, time since diagnosis, and undergoing systemic therapy, radiation or hormone treatment are known to be related to co-morbid CVD and/or HRQoL [Citation3,Citation4,Citation6,Citation16]. We therefore examined whether the relation between co-morbid CVD at cancer diagnosis and HRQoL differed by gender, age (≤65 vs. >65), time since diagnosis (0–5 years vs. >5 years), systemic therapy, radiation or hormone treatment by adding interaction terms (CVD*gender/age/time since diagnosis/systemic therapy/radiation/hormone treatment) together with their main effects to the model.
All these tests were two-sided and statistically significant if p < .05. We conducted exploratory multiple tests for the total sample of cancer survivors and in smaller subsamples for each malignancy separately. Therefore we did not choose to use a more stringent p-value of .01. Clinically meaningful differences for the EORTC QLQ‐C30 were determined using EORTC guidelines, which were developed by a systematic review of 152 cancer‐specific articles, expert opinions, and meta‐analyses [Citation17]. These EORTC guidelines classify four groups: Trivial, circumstances unlikely to have any clinical relevance or where there was no difference; Small, subtle but nevertheless clinically relevant; Medium, likely to be clinically relevant but to a lesser extent; Large, one representing unequivocal clinical relevance [Citation17]. Analyses were performed using IBM SPSS V24.0.
Results
Socio-demographics, clinical characteristics, and co-morbid CVD
In total, 5930 cancer survivors were included, with a median age of 61 years at cancer diagnosis, where little over half was male (n = 3421, 57.7%), . Nearly a quarter (n = 1441, 24.3%) had a co-morbid CVD at cancer diagnosis. Most prevalent CVD’s for the total group of 503 (8.5%) cancer survivors were a myocardial infarct, angina pectoris, or coronary artery disease. The second category was having cardiac arrhythmias with 272 (4.6%) cancer survivors. Thirdly, 183 (3.1%) of cancer survivors had a co-morbid peripheral arterial disease.
Table 1. Socio-demographics and clinical characteristics stratified by CVD status.
Cancer survivors with co-morbid CVD were more often male, older and had a lower educational level, were less often treated with surgery, systemic therapy and radiation, received more often hormonal treatment, had a shorter follow-up time (i.e. time between cancer diagnosis and HRQoL measurement) and reported more co-morbidities compared to those without CVD.
The sample of 5930 cancer survivors consisted of 2281 colorectal, 280 thyroid, 1054 prostate, 177 endometrium, 389 ovarian cancer, 212 melanoma, 874 non-Hodgkin lymphoma, 194 Hodgkin lymphoma, 242 CLL, and 227 MM survivors [Citation10]. CVD’s are most common among prostate (27.8%), CLL (26%), MM (21.7), non-Hodgkin lymphoma (20.3%), and colorectal cancer survivors (20.2%).
Co-morbid CVD and HRQoL
Total group
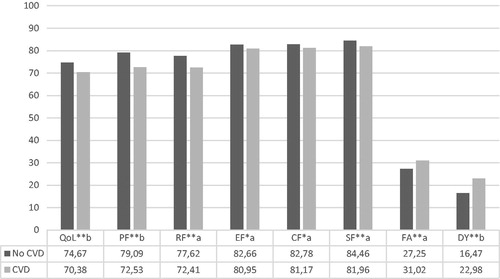
Cancer survivors who had a CVD at cancer diagnosis (n = 1441, 24.3%) reported significantly lower scores on global QoL and all functional scales, with more symptoms of fatigue and dyspnea (all p < .05) compared to those without CVD (). Differences in global QoL, physical functioning and dyspnea were of small clinical importance. The remaining HRQoL scales differences were significant yet of trivial clinical importance. Furthermore, the following socio-demographic and clinical characteristics were related to poorer global QoL and functioning, and more symptoms of fatigue and dyspnea: female gender, not being married or co-habiting, lower education, more co-morbidities, shorter time after diagnosis, and higher tumor stage. Older age was related to poorer physical functioning and more symptoms of fatigue, yet better global QoL and emotional, cognitive and social functioning.
Figure 1. Differences in HRQoL among those with and without CVD at cancer diagnosis for all survivors combined. Note: *p < .05, **p < .01. a: trivial; b: small; c: medium clinically important difference. QoL: global QoL; PF: physical functioning; RF: role functioning; EF: emotional functioning; CF: cognitive functioning; SF: social functioning; FA: fatigue; DY: dyspnea. Controlling for age, gender, marital status, education, stage, time since diagnosis, and co-morbidities.
Malignancy specific
The associations between co-morbid CVD at cancer diagnosis and HRQoL for each of the ten malignancies are presented in . To ensure sufficient power, effect modifications for gender, age, time since diagnosis and cancer treatment were performed in the three largest cancer survivor cohorts in which the prevalence of CVD was high enough: colorectal (n = 2281, n = 454 with CVD), prostate (n = 1054, n = 291 with CVD) and non-Hodgkin lymphoma survivors (n = 874, n = 175 with CVD).
Table 2. Unstandardized beta’s of linear regression analyses associating co-morbid CVD at cancer diagnosis with HRQoL scales specified for each malignancy.
Colorectal cancer
Colorectal cancer survivors with a co-morbid CVD at cancer diagnosis (n = 454, 20.2%) reported significantly lower scores, which were of small clinical important difference, on physical functioning (B = −6.89) and dyspnea (B = 5.75), all p < .05. Additionally, statistically significant yet difference of trivial clinical importance was found for global QoL (B = −3.54), role functioning (B = −3.51) and more symptoms of fatigue (B = 2.63).
There was a significant age-interaction effect for physical functioning (B = −6.63 p < .01). Stratified analysis showed that the negative relation between having a co-morbid CVD and physical functioning was stronger for older CRC survivors (B = −8.69, p < .01) than for younger CRC survivors (B = −5.30, p < .01). Additionally, for role functioning a significant interaction term was found for systemic therapy (B = 5.63, p < .01), where the negative relation between having a co-morbid CVD and role functioning was limited to the group of survivors who were not treated with systemic therapy (B = −4.26, p = .01). There were no significant interaction effects for gender, radiotherapy or time since diagnosis (p > .05).
Prostate cancer
Prostate cancer survivors with co-morbid CVD at cancer diagnosis reported lower scores of small clinical importance on global QoL (B = −4.67), physical (B = −6.77) and role functioning (B = −7.20), and higher scores on fatigue (B = 5.06) and dyspnea (B = 8.26) compared to those without CVD (p < .01). Additionally a significant but clinically trivial relation was found between having co-morbid CVD and social functioning (B= −4.18).
Significant age-interaction effects were found for global QoL (B = 6.17, p = .02) and emotional functioning (B = 6.48, p = .02). There was a significant negative relation between having co-morbid CVD on global QoL and on emotional functioning, which was limited to younger prostate cancer survivors (global QoL: B = −8.54, p < .01 and emotional functioning: B = −5.93, p < .01). There were no significant interaction effects for radiotherapy or time since cancer diagnosis.
Non-Hodgkin lymphoma
A small clinically important difference was found for physical functioning (B = −6.29, p < .01), with lower scores for those who had co-morbid CVD. Non-Hodgkin lymphoma survivors with CVD reported more symptoms of dyspnea (B = 11.58, p < .01), which was of medium clinical importance. Furthermore, two significant but clinically trivial differences were found; non-Hodgkin lymphoma survivors with co-morbid CVD at cancer diagnosis reported lower scores for global QoL (B = −3.71) and role functioning (B = −5.94), p < .05.
The relation between having a co-morbid CVD at cancer diagnosis and the symptom dyspnea differed by gender (interaction B = 9.90, p = .03), as there was only a significant positive relation among females (B = 16.98, p < .01). Additionally, the relation between having co-morbid CVD and global QoL differed by being treated with radiotherapy or not (interaction B = 9.90, p = .03). Stratified analyses showed that co-morbid CVD was only significantly related to global QoL among those who were treated with radiotherapy (B = −12.07, p < .01). No significant interaction effects for age, systemic therapy or time since diagnosis were found (p > .05).
Ovarian cancer
Ovarian cancer survivors with co-morbid CVD at cancer diagnosis reported more symptoms of dyspnea (B = 8.50, p = .03), which was of small clinical importance.
Melanoma
A small clinically significant effect was found, with lower scores on cognitive functioning (B = −8.46, p = .03) among melanoma survivors who had co-morbid CVD at cancer diagnosis.
CLL
CLL survivors with a co-morbid CVD at cancer diagnosis reported small clinically important lower scores on global QoL (B = −9.03), physical (B= −13.80), role (B = −10.58), emotional (B = −7.58), and social functioning (B = −9.24), yet higher scores on symptoms of fatigue (B = 10.42) (all p < .05).
Thyroid and endometrium cancer, Hodgkin lymphoma and MM
No significant relations between co-morbid CVD at cancer diagnosis and HRQoL were seen among thyroid, endometrium, Hodgkin lymphoma, and MM survivors.
Discussion
Of the total sample of cancer survivors (n = 5930), 24.3% (n = 1441) had a co-morbid CVD at the time of their cancer diagnosis. A co-morbid CVD-diagnosis was most common among prostate (27.8%), CLL (26%), MM (21.7), non-Hodgkin lymphoma (20.3%), and colorectal cancer survivors (20.2%). Overall, cancer survivors with co-morbid CVD were more often male, older and had a lower educational level, received less often treatment (surgery, systemic therapy, and radiation, yet more often hormonal treatment), and reported more other co-morbidities compared to those without CVD.
For the total group, survivors with CVD at cancer diagnosis reported statistically and clinically significant lower scores on global QoL and physical functioning and higher scores for dyspnea (p < .05) compared to those without CVD. Among colorectal, prostate, ovarian cancer, non-Hodgkin lymphoma and CLL survivors, those with a co-morbid CVD at cancer diagnosis reported small clinically relevant lower scores on global QoL, physical and role functioning and more symptoms of fatigue and dyspnea. This finding is consistent with studies among CVD patients, often experiencing impairments in global QoL, physical and role functioning [Citation18,Citation19]. Furthermore, fatigue and dyspnea are known to be key symptoms of various CVDs [Citation20]. Additionally, survivors with co-morbid CVD at cancer diagnosis reported worse emotional (CLL), social (prostate and CLL), and cognitive functioning (melanoma). As CLL cannot be cured and following guidelines treatment options are highly dependent on overall health, having a co-morbid CVD at cancer diagnosis could result in impairments in emotional and social functioning. A well-known long-term problem among prostate cancer survivors is incontinence which has a negative impact on social functioning [Citation21]. Common treatment among CVD patients are diuretics to reduce strain on the heart by increasing urine production. This could explain why prostate cancer survivors with a co-morbid CVD at cancer diagnosis report lower social functioning. Reduced cognitive functioning among melanoma survivors is understandable as these cancer survivors suffered from vascular CVDs often located in the brain such as a cerebrovascular accident as stated in the NCR (data not shown). No significant relations between co-morbid CVD and HRQoL were found among thyroid and endometrium cancer, Hodgkin lymphoma and MM survivors. This could be due to the small number of survivors with co-morbid CVD (thyroid (n = 15), endometrium (n = 28) cancer, Hodgkin lymphoma (n = 12), and MM (n = 48)).
Among non-Hodgkin lymphoma survivors, the relation between having co-morbid CVD and symptoms of dyspnea was limited to women. This is in line with the clinical presentation, as dyspnea is a typical symptom displayed and reported by women [Citation22]. However, this gender effect was not seen among colorectal cancer survivors. Age-modification effects were seen among colorectal and prostate cancer survivors. That is, the negative relation between co-morbid CVD at cancer diagnosis and physical functioning was stronger among older colorectal cancer survivors (>65 years). Contrary, only younger prostate cancer survivors (≤65 years) reported lower global QoL and emotional functioning when they had a co-morbid CVD at cancer diagnosis. This is in line with general findings relating older age to poorer physical functioning [Citation23], and younger age to poorer emotional functioning [Citation24]. Additionally, the effect of co-morbid conditions at younger age may have a stronger impact on global QoL than among older survivors. This could be because overall HRQoL decreases due to aging and the development of co-morbid conditions is somewhat expected and similar to their non-cancer peers. Additionally, younger survivors have more competing responsibilities, hence the negative relation between co-morbid CVD and HRQoL is more severe as has been previously described [Citation8].
The negative relation of having co-morbid CVD at cancer diagnosis and role functioning was limited to colorectal cancer survivors who were not treated with systemic therapy. Systemic treatment can have serious long-term adverse effects [Citation25] and is known to have a negative impact on HRQoL. In the case of both having a co-morbid CVD and being systemically treated for cancer, the impact of having co-morbid CVD at cancer diagnosis may be secondary to the negative impact of systemic treatment when it comes to HRQoL. In other words, only when the impact of systemic treatment on HRQoL is excluded, one sees a negative relation between having a co-morbid CVD and HRQoL. Non-Hodgkin lymphoma survivors with co-morbid CVD at cancer diagnosis reported lower global QoL, only if they were treated with radiotherapy. It could be that among non-Hodgkin lymphoma survivors with co-morbid CVD, radiation to the chest leads to an increase in cardiovascular problems[Citation3,Citation4], especially among those who had preexisting CVDs. In turn this could negatively impact their global QoL.
Examined among colorectal, non-Hodgkin lymphoma, and prostate cancer survivors we found that the negative relation between having a co-morbid CVD at cancer diagnosis and HRQoL was not related to the timing of the HRQoL questionnaire. This is inconsistent with a previous study among breast cancer survivors, where the relation between co-morbidities present at the time of the assessment and HRQoL was stronger when the cancer diagnosis was longer ago [Citation8]. In the current study, we have no information on date of CVD diagnosis only whether cancer survivors have a co-morbid CVD at cancer diagnosis. Hence, it is unclear how relevant the co-morbid CVD is when it comes to HRQoL at the time of the HRQoL-assessment often years later. The data did include information on whether cancer survivors were diagnosed with a secondary cancer during follow-up. We, therefore, performed sensitivity analyses examining the relation between co-morbid CVD at cancer diagnosis and HRQoL for this subgroup (n = 1100, 18.5%). Results showed similar regression coefficients as for the total group of survivors, yet some associations became non-significant which is likely due to reduced power (data not shown).
The following study limitations should be taken into account. No information is available on the severity of co-morbid CVD. Additionally, we do not have information on the development of new CVDs during follow-up or whether CVDs have worsened. Moreover, by virtue of remaining disease-free, long-term cancer survivors have a better prognosis, may have received less aggressive treatment likely resulting in a better HRQoL. Furthermore, the sample selection may have biased our results as non-respondents are more often male, younger (<60) or older (>70), have a lower socioeconomic status, received less often radiotherapy or no treatment and reported fewer co-morbidities [Citation10]. Finally, we have no information on HRQoL levels prior to the cancer diagnosis. It is likely that those cancer survivors with a co-morbid CVD at cancer diagnosis started out with a lower HRQoL prior to cancer diagnosis, as CVD is negatively related to HRQoL [Citation18,Citation19]. It may be the negative impact of having a CVD at cancer diagnosis that is responsible for the lower HRQoL levels at follow-up. In other words, the negative impact of cancer and its treatment on HRQoL might, in fact, be similar for both survivors with and without co-morbid CVD at cancer diagnosis. Alternatively, there may be a synergistic effect of having a co-morbid CVD and then additionally getting cancer and being treated for it. Hence, the impact of co-morbid CVD may result in a larger negative impact of cancer and its treatment on survivors’ HRQoL. Future research including information on HRQoL levels at the time of cancer diagnosis; follow-up data on the severity and development of CVD; and the inclusion of a third sample of patients with only CVD will provide insight into the individual and possible synergistic effects of cancer(treatment) and CVD on HRQoL.
A major strength is the large population-based sample of cancer survivors with various malignancies and the usage of the high-quality databases NCR and the PROFILES registry. This study, therefore, encompasses information across malignancies, with a wide age range, including men and women, short and long-term survivors, and survivors treated with a wide range of treatments. This allowed testing effect modifications for age, gender, time since cancer diagnosis, and systemic therapy, radiation, and hormone treatment. However, no detailed information on treatment such as type of medication or dosage was available. Furthermore, CVD status was retrieved from the NCR based on medical records which are generally regarded as reliable and complete sources of information on the patient's past and current health status [Citation26]. Nevertheless, only information on presence of CVD and not date of diagnosis was registered. Additionally, this is to our knowledge the first large study that examined the relation between having co-morbid CVD at cancer diagnosis and HRQoL.
In conclusion, co-morbid CVD at cancer diagnosis is negatively related to HRQoL among cancer survivors. Most consistent findings were found for global QoL, physical and role functioning, and the symptoms of fatigue and dyspnea. Health-care providers should, therefore, pay extra attention to this vulnerable group of cancer survivors with co-morbid CVD at cancer diagnosis when it comes to their HRQoL, even years after cancer treatment is finished. Additionally, more research focusing on unraveling mechanisms leading up to these HRQoL impairments is needed. Hence, differences in received treatment should be further explored, as co-morbid CVD can interfere with cancer treatment and prognosis [Citation27]. Cancer survivors with co-morbid CVDs may be undertreated [Citation6]. Furthermore, information on the severity and possible progression of CVDs should be taken into account as cancer treatment can be cardiotoxic inducing and worsening CVD [Citation3,Citation4].
Acknowledgments
Authors would like to acknowledge all contributing participants.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Ogle KS, Swanson GM, Woods N, et al. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88:653–663.
- Janssen-Heijnen ML, Szerencsi K, van de Schans SA, et al. Cancer patients with cardiovascular disease have survival rates comparable to cancer patients within the age-cohort of 10 years older without cardiovascular morbidity. Critic Rev Oncol/Hematol. 2010;76:196–207.
- Geiger S, Lange V, Suhl P, et al. Anticancer therapy induced cardiotoxicity: review of the literature. Anticancer Drugs. 2010;21:578–590.
- Schimmel KJ, Richel DJ, van den Brink RB, et al. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30:181–191.
- Lenihan DJ, Cardinale D, Cipolla CM. The compelling need for a cardiology and oncology partnership and the birth of the International CardiOncology Society. Prog Cardiovasc Dis. 2010;53:88–93.
- Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Critic Rev Oncol/Hematol. 2005;55:231–240.
- Vissers PA, Thong MS, Pouwer F, et al. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: analyses of data from the PROFILES registry. J Cancer Surviv. 2013;7:602–613.
- Schoormans D, Czene K, Hall P, et al. The impact of co-morbidity on health-related quality of life in breast cancer survivors and controls. Acta oncologica. 2015;54:727.
- van de Poll-Franse LV, Horevoorts N, van Eenbergen M, et al. The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47:2188–2194.
- de Rooij BH, Ezendam NPM, Mols F, et al. Cancer survivors not participating in observational patient-reported outcome studies have a lower survival compared to participants: the population-based PROFILES registry. Qual Life Res. 2018;27:3313.
- De Marco MF, Janssen-Heijnen ML, van der Heijden LH, et al. Comorbidity and colorectal cancer according to subsite and stage: a population-based study. Eur J Cancer. 2000;36:95–99.
- Schouten LJ, Jager JJ, van den Brandt PA. Quality of cancer registry data: a comparison of data provided by clinicians with those of registration personnel. Br J Cancer. 1993;68:974–977.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Hoboken, NJ, USA: John Wiley & Sons; 2011.
- Sangha O, Stucki G, Liang MH, et al. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163.
- Arndt V, Koch-Gallenkamp L, Jansen L, et al. Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta oncologica. 2017;56:190–197.
- Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. JCO. 2011;29:89–96.
- Brown N, Melville M, Gray D, et al. Quality of life four years after acute myocardial infarction: short form 36 scores compared with a normal population. Heart. 1999;81:352–358.
- Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87:235–241.
- Leuzzi C, Sangiorgi GM, Modena MG. Gender-specific aspects in the clinical presentation of cardiovascular disease. Fundam Clin Pharmacol. 2010;24:711–717.
- Fleshner N, Herschorn S. The artificial urinary sphincter for post-radical prostatectomy incontinence: impact on urinary symptoms and quality of life. J Urol. 1996;155:1260–1264.
- Garcia M, Mulvagh SL, Merz CN, et al. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118:1273–1293.
- Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37:1345–1351.
- Netuveli G, Blane D. Quality of life in older ages. Br Med Bull. 2008;85:113–126.
- Angelis CD. Side effects related to systemic cancer treatment: are we changing the Promethean experience with molecularly targeted therapies? Curr Oncol. 2008;15:198–199.
- Kieszak SM, Flanders WD, Kosinski AS, et al. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52:137–142.
- Lemmens VE, Janssen-Heijnen ML, Verheij CD, et al. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg. 2005;92:615–623.
