Abstract
Background: Elevated neutrophil-lymphocyte ratio (NLR) and hyponatremia each predict poor prognosis in renal cell carcinoma (RCC). Since no previous studies have looked at the combined effect of these two prognostic markers, we examined how NLR and hyponatremia combined associates with mortality and hypothesized that elevated NLR and hyponatremia at RCC diagnosis and at RCC recurrence indicate poorer prognosis.
Material and methods: Using Danish medical registries 1999–2015, we included 970 patients from two regions with incident RCC and a measurement of NLR and sodium. NLR was categorized as ≤3.0 and >3.0 and sodium as < lower limit of normal (LLN) and ≥ LLN. Outcomes were survival after RCC diagnosis and first recurrence, respectively. We estimated absolute survival and hazard ratios (HR) using multivariate Cox regression.
Results: At RCC diagnosis, 559 (57.6%) had NLR >3.0 and 240 (24.7%) had hyponatremia, the 5 year-survival rate was 35.2% in NLR > 3.0 vs. 69.2% in NLR ≤3.0, adjusted HR 1.8 (95% confidence intervals (CI), 1.4; 2.2). In patients with NLR >3.0 and concomitant hyponatremia vs. NLR ≤3.0 and normal sodium the 5-year survival rate was 21.7% vs. 73.2%, adjusted HR 2.8 (95% CI, 2.1; 3.8). At RCC recurrence, patients with NLR >3.0 and hyponatremia similarly had poorest survival, adjusted HR 3.6 (95% CI, 1.0; 12.8).
Conclusion: Elevated NLR alone and in combination with hyponatremia at time of initial RCC diagnosis and at time of RCC recurrence are associated with poor prognosis. Combining these two prognostic markers yield a stronger association than NLR considered alone. This may impact prognostic prediction and its related therapeutic strategy.
Introduction
Patients with renal cell carcinoma (RCC) have overall five-year survival rates of 60%–70% [Citation1]. Several tumor pathology and patient-related factors affect RCC prognosis. Well-established prognostic models for both localized [Citation2,Citation3] and metastatic [Citation4,Citation5] RCC have incorporated these factors. The TNM staging system, Fuhrman grade and patients’ performance status are combined in the UCLA integrated prognostic model for localized disease [Citation3] and T stage, N stage, tumor size, Fuhrman grade and presence of tumor necrosis are combined in Leibovich prognostic index, also used in localized RCC [Citation2]. Concerning metastatic RCC (mRCC), a widely used prognostic model is the MSKCC/Motzer score [Citation4] comprising poor performance status (Karnofsky score <80%), serum calcium level >2.5 mmol/L, serum LDH level >1.5 × ULN, hemoglobin level < LLN, and time from diagnosis to start of systemic treatment <1 year. Other well-established prognostic models for mRCC includes the Heng score linking elevated platelet and neutrophil count, anemia, hypercalcemia, poor performance status and time from diagnosis to start of systemic treatment <1 year with shorter survival in patients treated with VEGF–targeted therapy [Citation5]. Identification and incorporation of additional biomarkers may improve the prognostic information.
Inflammation is known to have impact on several steps in tumor development [Citation6,Citation7] and inflammatory markers, such as neutrophil-lymphocyte ratio (NLR), have been demonstrated as prognostic factors in various malignancies [Citation8], including localized [Citation9,Citation10] and metastatic [Citation10–12] RCC. An elevated NLR indicates chronic inflammation, favoring tumor growth, as well as reduced lymphocyte antitumor immune response; this may contribute to a more aggressive tumor biology with more aggressive histologic subtypes, higher Fuhrman grade and higher T stage at time of nephrectomy [Citation13,Citation14], and may contribute to tumor progression and poor prognosis [Citation9].
A new strong biomarker is hyponatremia. A diagnosis of hyponatremia in non-cancer cohorts was followed by a markedly increased risk of a subsequent cancer diagnosis, including kidney cancer [Citation15,Citation16]. Hyponatremia is associated with poor prognosis in several medical conditions and in several cancer diseases, including localized [Citation17] and metastatic [Citation18–22] RCC. Although hyponatremia is a very common condition and may occur due to reduced kidney function, the mechanisms underlying development of hyponatremia in RCC patients is not completely understood, but an association with chronic inflammation has been suggested [Citation19,Citation23]. As suggested in the editorial by Bellmunt, chronic inflammation may lead to production of interleukin 6 (IL-6), which can stimulate excessive release of antidiuretic hormone, resulting in hyponatremia. No previous studies have looked at the combined prognostic effect of NLR and hyponatremia in RCC patients. Therefore, using data from Danish medical registries, we evaluated in a large RCC cohort the prognostic impact of NLR and hyponatremia, and whether combining NLR and hyponatremia improved prognostic ability, in a large population-based cohort study at time of initial diagnosis and at time of RCC recurrence.
Material and methods
Data sources and study population
We performed a population-based cohort study including all patients above 18 years of age diagnosed with localized or metastatic RCC in 1999–2015 in the North and Central Denmark Regions (representing one-third of Denmark’s population). The Danish health care system provides free tax-funded health care to all residents and the Danish Civil Registration System (CRS) assigns a unique ten-digit civil person registration (CPR) number to all inhabitants in Denmark at birth or upon immigration. This number is used in all Danish registries and allows unambiguous linkage of information from multiple data sources [Citation24]. We linked data from the CRS to the Danish National Pathology Registry (DPR), the Danish Cancer Registry (DCR), the Danish National Patient Registry (DNPR) and the Clinical Laboratory Information System Research Database (LABKA).
DPR includes descriptions of pathological specimens analyzed in Denmark using Danish SNOMED codes [Citation25]. We identified our study population based on these SNOMED codes (see Supplementary Appendix A) defining date of diagnosis as date of pathology requisition. DCR provided information on tumor stage. DNPR provided information regarding date of hospital admission and discharge, diagnosis types (ICD-10 codes), and type of surgery and treatment. LABKA [Citation26] provided information on laboratory analyses from 30 days before RCC diagnosis and onwards. CRS [Citation24] provided information on sex, birthdates, emigration and vital status.
NLR and sodium values
We defined NLR, our primary exposure, as the absolute neutrophil count (×109/L) divided by the absolute lymphocyte count (×109/L) assessed within 30 days before diagnosis. If several values existed in this 30 day-period, we used the latest before date of diagnosis. We predefined cutoff as 3.0, with an elevated NLR being >3.0 and low NLR ≤3.0.
Serum sodium levels were similarly assessed and categorized according to normal values for a person above 18 years (137–145 mmol/L). We defined hyponatremia as values below lower limit of normal (LLN). We excluded patients without a NLR and sodium measurement in this 30-days time period.
Likewise, we obtained NLR and sodium measures within 30 days before date of RCC recurrence. We defined recurrence as first occurrence of either a RCC recurrence diagnosis recorded in DPR, or a hospital contact to an oncology department with a kidney cancer diagnosis more than 120 days after date of nephrectomy and within 3 years of date of RCC diagnosis.
Survival
Primary outcomes were 1- and 5-year survival after initial RCC diagnosis. Among patients with localized disease at time of initial RCC diagnosis who underwent curative-intended surgery, secondary outcomes were 1- and 3-year survival after first RCC recurrence.
Covariates
Based on existing literature we included the following covariates: sex, age (≤60 vs. >60 years), Charlson Comorbidity Index (CCI) score (0, 1–2 and >3), TNM stage (I–IV), histological subtypes (clear cell and non-clear cell), tumor size (<4, 4–7 and >7 cm), Fuhrman grade (1–2 vs. 3–4), presence of tumor necrosis or sarcomatoid differentiation, Leibovich score (0–2, 3–5 and >6) and selected baseline biochemical values including the IMDC factors hemoglobin, platelets and calcium and additionally sodium, LDH, CRP and albumin stratified according to the upper or lower limits of normal (ULN and LLN) (see Supplementary Appendix B).
Statistical analysis
Patients’ characteristics at time of RCC diagnosis were described according to NLR status (elevated (>3.0) or low (≤3.0)). We followed all patients five years from date of diagnosis or until death, emigration or end of study period (31 December 2015).
We estimated survival probabilities using the Kaplan-Meier method and estimated hazard ratios (HR) with 95% confidence intervals (95% CI) using Cox regression. A priori we selected three multivariate models: (1) Adjusting for age, sex, stage and Charlson Comorbidity, (2) also including albumin, sodium, calcium and LDH and (3) including hemoglobin, CRP, and platelets as well. Additionally, we restricted the analyses to (1) patients with confirmed clear cell RCC and (2) patients with localized RCC at time of initial diagnosis, respectively.
In the final analyses, we did not include tumor size, Fuhrman grade, Leibovich score, and presence of tumor necrosis or sarcomatoid differentiation due to a high prevalence of missing data. For the remaining covariates, missing data were analyzed according to the missing indicator method and we explored the impact of having missing data in our NLR analyses with a sensitivity analysis comparing our results with a worst-case scenario categorizing all missing values among patients with NLR ≤3.0 as the best prognostic categorical value, whereas missing values among patients with NLR >3.0 were categorized as the worst prognostic value.
We assessed the assumption of proportional hazards by log-minus-log plots and unless stated otherwise, we found it not to be violated. All statistical analyses were conducted using STATA version 14.2 software package. The study was approved by the Danish Data Protection Agency (Jr. number: 2014-54-0922). In Denmark, approval from the Research ethics Committees is not needed for purely registry-based observational studies.
Results
Among 2849 patients with a pathology verified RCC diagnosis from 1999 to 2015, 970 patients had a recorded NLR and sodium value within 30 days of RCC diagnosis and were included in the study. We had virtually complete follow-up (one person emigrated before end of follow-up) with a median follow-up of 2.3 years (range, 0.7–4.3 years). Median age at time of diagnosis was 66.6 years. Included patients had similar distribution of sex, age, and comorbidity level as those not included due to missing data (data not shown).
Median NLR at diagnosis was 3.4. Overall, 411 had NLR ≤3.0 and 559 had NLR >3.0. Patients with elevated NLR were older, more often men, more likely to have stage IV disease, had higher Charlson Comorbidity, were less likely to have clear cell RCC, and less likely to have surgery (). Patients with elevated NLR tended to have low hemoglobin, sodium and albumin levels and high calcium, LDH, CRP and platelet levels ().
Table 1. Clinical and pathological characteristics of RCC patients stratified according to NLR level.
In total, 240 had hyponatremia and 730 had normal sodium values. Apart from sex distribution, patients with hyponatremia had similar distributions regarding the above-mentioned clinical and pathological characteristics (supplemental table 1).
Survival at initial RCC diagnosis
During follow-up, 436 (45.0%) patients died. The 1-year survival rate was 59.5% in RCC patients with NLR >3.0 compared with 87.7% in patients with low NLR and 5-year survival rates were 35.2% and 69.2%, respectively (. Compared with NLR ≤3.0, an elevated NLR at time of RCC diagnosis was associated with poor prognosis, unadjusted HR = 3.1 (95% CI, 2.5; 3.9), and sex, age, stage and Charlson comorbidity adjusted HR = 2.4 (95% CI, 1.9; 3.0). Even after adjusting for markers of inflammatory activity (hemoglobin, platelets and CRP), NLR remained associated with an almost two-fold increased mortality (HR = 1.8 (95% CI, 1.4; 2.2)) (). Re-analyzing data with different NLR cutoff values ranging between 2.0 and 5.0 emphasized these findings (Supplemental table 2). Examining the impact of having missing data in a worst case scenario substantially attenuated the association, adjusted for all covariates HR was 1.1 (95% CI, 0.8; 1.4).
Figure 1. Kaplan-Meier curves for overall survival according to (A) NLR and (B) NLR and serum sodium groups at time of initial RCC diagnosis.
NLR: neutrophil-lymphocyte ratio; RCC: renal cell carcinoma; LLN: lower limit of normal.
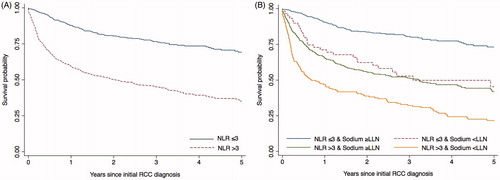
Table 2. The association between NLR at time of initial RCC diagnosis and mortality.
In patients with clear cell RCC, the association was slightly stronger in each adjusted analysis while patients with localized RCC had similar estimates as in our main analysis (). In the stratified analyses (), elevated NLR was associated with a nearly two-fold higher mortality in patients with low hemoglobin at time of initial diagnosis and a nearly three-fold increased mortality in patients who presented with normal hemoglobin level, even after taking age, sex, stage and comorbidity into account. Although less pronounced, stratifying by low CRP or low platelets level resulted in different stratum-specific estimates as well ().
Table 3. The association between NLR at time of initial RCC diagnosis and mortality. Results from stratified analysis.
Combining NLR and serum sodium
At time of RCC diagnosis; 181 patients had elevated NLR and hyponatremia and 352 patients had NLR ≤3.0 and no hyponatremia. We found that 25% of patients with an elevated NLR in combination with hyponatremia at time of RCC diagnosis died within 2-3 months, and the vast majority of patients died within 5 year; the 5-year survival rate was 21.7% in RCC patients with an elevated NLR and hyponatremia compared with 73.2% in patients with NLR ≤3.0 and no hyponatremia (. The corresponding unadjusted HR was 5.6 (95% CI, 4.2; 7.4) and age, sex, stage and Charlson comorbidity adjusted HR was 4.1 (95% CI, 3.1; 5.5) (). Patients with either high NLR or hyponatremia alone had 5-year survival rates of 35.2% and 27.0%, respectively.
Table 4. The combined prognostic effect of NLR and serum sodium at time of initial RCC diagnosis.
Survival at RCC recurrence
Of the 442 localized RCC patients who underwent surgery, 76 patients (17.2%) had RCC recurrence within three years of follow-up. Of these, 58 had NLR and sodium measured at time of diagnosis with RCC recurrence and was included in analysis. To obtain proportional hazards, we restricted follow-up to two years.
In total, 32 (55.2%) patients had a NLR ≤3.0 and 26 (44.8%) had a NLR >3.0 at time of RCC recurrence. The 1-year survival rate was 65.2% in RCC patients with elevated NLR compared with 84.4% in patients with low NLR, and 2-year survival rates were 53.0% and 78.0%, respectively. An elevated NLR at RCC recurrence was associated with poorer prognosis with unadjusted HR = 2.6 (95% CI, 1.0; 6.6) and adjusted HR = 2.3 (95% CI, 0.9; 5.9) (adjusting for sex and age at time of RCC recurrence).
At RCC recurrence, 11 patients had elevated NLR and hyponatremia and 29 patients had NLR ≤3.0 and no hyponatremia. Patients with hyponatremia and elevated NLR at RCC recurrence had a 1-year survival rate on 62.3% compared with 86.2% in patients without hyponatremia and NLR ≤3.0, and a 2-year survival rate on 52.0% compared with 82.6%, respectively. The unadjusted HR was 3.7 (95% CI, 1.1; 12.9) and adjusted for age and sex, HR was 3.6 (95% CI, 1.0; 12.8).
Discussion
Elevated NLR alone and in combination with hyponatremia at time of initial RCC diagnosis and at RCC recurrence were associated with poor prognosis, and combining these two features yielded an even stronger association than NLR alone. Still, when adjusting for several individual, pathological and biochemical covariates, including some IMDC factors, the association remained elevated. Restricting the analysis to localized disease at time of initial diagnosis, patients with elevated NLR likewise had poorer prognosis. While none of previous studies have looked at the combined effect of elevated NLR and hyponatremia, several previous studies have shown each of these factors as prognostic markers in both localized and metastatic RCC [Citation9–11,Citation17–21,Citation27]. Our study is also the first to follow the same cohort of patients from localized RCC diagnosis to recurrence, and to demonstrate the poorer prognosis of combined NLR-hyponatremia measurement at both time points.
Kidney cancer is among the 10 most common cancers in both men and women worldwide with RCC being the most common histologic subtype accounting for approximately 90% of primary kidney tumors [Citation28]. Despite progress in RCC survival rates, there is still a relatively high mortality. Current well-known prognostic models are largely based on postoperative pathologic analysis of the surgical specimen and although such tumor-related factors are consistent predictors of outcome at a population level, they are not always reliable in individual patients. Incorporation of more individualized factors, for an example biomarkers that may be induced in the patient due to the presence of the tumor, such as elevated NLR and hyponatremia, may be valuable and could improve the prognostic information [Citation29,Citation30]. NLR and sodium are readily available, at low cost, and may thus serve as cost-effective markers for prognostic prediction; they have the potential to be easily incorporated in prognostic models to optimize outcome prediction. Moreover, NLR and hyponatremia may be incorporated as stratification factors in clinical trial design. Our results showed a short-term mortality as high as 25% within few months after initial RCC diagnosis in patients with elevated NLR and hyponatremia, and the majority of these patients died within 5 years; this notion may impact follow-up and patient counseling.
Existing studies have used various NLR cutoffs ranging between 2.0 and 5.0 [Citation10,Citation11]. From the point of clinical practice, using an established and validated fixed cutoff is preferred for offering patients a simple risk classification into ‘high’ versus ‘low’. However, the fact that changing the cutoff in our data within the range of 2.0–5.0 did not alter the conclusion that an elevated NLR was associated with poorer survival suggest that using 3.0 as a fixed threshold may be too rigor since patients with NLR levels between 2.0 and 3.0 also had an increased mortality compared with those with even lower NLR. The interpretation is that the NLR measurement strongly mirrors two important hallmarks of cancer, both ‘tumor-promoting inflammation’ and ‘avoiding immune destruction’ [Citation7]. One may speculate whether changes in NLR over time may offer better prognostic information [Citation20,Citation31]. Unfortunately, our data did not allow us to examine this any further.
Hyponatremia is a strong, prognostic feature. Our data showed hyponatremia not only linked to underlying chronic inflammation, as previously suggested [Citation23]. In patients with poor prognostic features, i.e., anemia, thrombocytopenia, elevated NLR, the addition of hyponatremia provided even further poor prognostic information. Previous research have demonstrated the development of hyponatremia as an overall negative prognostic signal for various conditions [Citation21], e.g., liver cirrhosis patients on waiting list for liver transplantation [Citation32] or childhood meningitis [Citation33]; hyponatremia was associated with increased mortality. Our data are in line with this and in line with previous findings in localized and metastatic RCC patients [Citation18,Citation20]. Our findings of a higher mortality among RCC patients with hyponatremia should prompt further research in this area. Hyponatremia may be linked to illness in general, since patients with RCC often have comorbidity. However, after adjusting for comorbidity, the association still remained elevated. Furthermore, hyponatremia may occur due to reduced kidney function caused by RCC. Since we did not have data on kidney function, we could not examine this further. However, the patients who had a nephrectomy, all had their date of surgery after the diagnosis of renal cell cancer, accordingly, serum sodium, which was measured up to 30 days before diagnosis, was measured before date of surgery and therefore, sodium levels is not suspected to be influenced by kidney surgery.
The main strengths of this study are its population-based design using region- and nationwide administrative and medical registries with prospectively collected data preventing selection bias and allowing for a virtually complete follow-up of all patients. Another strength is the relatively large sample size. Limitations are that our dataset may have missing recurrences and missing data on some pathology features, which means that we could not take tumor size, Fuhrman grade, Leibovich score and presence of tumor necrosis or sarcomatoid differentiation into account as potential confounding factors. Unfortunately, since no other medical registries have information on these pathology characteristics, it is not possible to address this further. These pathology characteristics are missing due to the simple fact that the pathologist has not registered these features. The Danish Renal Cancer (DaRenCa) multidisciplinary group has encouraged improved registration to national registries. Moreover, we did not have access to information regarding concomitant medication, time to systemic therapy, performance status and smoking status. However, we addressed missing data among the included covariates using the missing indicator method, and find it unlikely that missing data entirely explains the increased mortality in our study. Although we cannot entirely rule out selection bias due to missing data, we find it reassuring that patients excluded due to missing data had similar distribution of sex, age and level of comorbidity.
In conclusion, elevated NLR alone and in combination with hyponatremia at time of initial RCC diagnosis and at time of RCC recurrence were associated with poor prognosis. Combining these two prognostic markers yield a stronger association than NLR considered alone. This may impact patient counseling and clinical treatment decisions.
Supplemental Material
Download (130.7 KB)Disclosure statement
The authors report no conflicts of interest. The named funding sources had no role in any process during the research period and manuscript preparation.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
- Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–1671.
- Patard J-J, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an International Multicenter Study. J Clin Oncol. 2004;22:3316–3322.
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540.
- Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;127:5794–5799.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
- Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200–207.
- Byun S-S, Hwang EC, Kang SH, et al. Prognostic significance of preoperative neutrophil-to-lymphocyte ratio in nonmetastatic renal cell carcinoma: a large, multicenter cohort analysis. Biomed Res Int. 2016;2016:5634148.
- Hu K, Lou L, Ye J, et al. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open. 2015;5:e006404.
- Na N, Yao J, Cheng C, et al. Meta-analysis of the efficacy of the pretreatment neutrophil-to-lymphocyte ratio as a predictor of prognosis in renal carcinoma patients receiving tyrosine kinase inhibitors. Oncotarget. 2016;7:44039–44046.
- Templeton AJ, Knox JJ, Lin X, et al. Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol. 2016;70:358–364.
- Viers BR, Thompson RH, Lohse CM, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts tumor pathology in newly diagnosed renal tumors. World J Urol. 2016;34:1693–1699.
- Otunctemur A, Dursun M, Besiroglu H, et al. Clinical significance of preoperative neutrophil - to - lymphocyte ratio in renal cell carcinoma. Int Braz J Urol. 2016;42:678–684.
- Holland-Bill L, Christiansen CF, Farkas DK, et al. Diagnosis of hyponatremia and increased risk of a subsequent cancer diagnosis: results from a nationwide population-based cohort study. Acta Oncol. 2018;57:522–527.
- Selmer C, Madsen JC, Torp-Pedersen C, et al. Hyponatremia, all-cause mortality, and risk of cancer diagnoses in the primary care setting: a large population study. Eur J Intern Med. 2016;36:36–43.
- Vasudev NS, Brown JE, Brown SR, et al. Prognostic factors in renal cell carcinoma: association of preoperative sodium concentration with survival. Clin Cancer Res. 2008;14:1775–1781.
- Jeppesen AN, Jensen HK, Donskov F, et al. Hyponatremia as a prognostic and predictive factor in metastatic renal cell carcinoma. Br J Cancer. 2010;102:867–872.
- Kawashima A, Tsujimura A, Takayama H, et al. Impact of hyponatremia on survival of patients with metastatic renal cell carcinoma treated with molecular targeted therapy. Int J Urol. 2012;19:1050–1057.
- Schutz FAB, Xie W, Donskov F, et al. The impact of low serum sodium on treatment outcome of targeted therapy in metastatic renal cell carcinoma: results from the international metastatic renal cell cancer database consortium. Eur Urol. 2014;65:723–730.
- Penttilä P, Bono P, Peltola K, et al. Hyponatremia associates with poor outcome in metastatic renal cell carcinoma patients treated with everolimus: prognostic impact. Acta Oncol. 2018;57:1580–1585.
- Soerensen AV, Geertsen PF, Christensen IJ, et al. A five-factor biomarker profile obtained week 4-12 of treatment for improved prognostication in metastatic renal cell carcinoma: results from DARENCA study 2. Acta Oncol. 2016;55:341–348.
- Bellmunt J, Leow JJ. Hyponatremia associated with worse outcomes in metastatic renal cell cancer: a potential target for intervention? Eur Urol. 2014;65:731–732.
- Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549.
- Erichsen R, Lash TL, Hamilton-Dutoit SJ, et al. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and Data Bank. Clin Epidemiol. 2010;2:51–56.
- Grann AF, Erichsen R, Nielsen AG, et al. Existing data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin Epidemiol. 2011;3:133–138.
- Keizman D, Ish-Shalom M, Huang P, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48:202–208.
- Cancer.org [Internet]. American Cancer Society. [cited 2019 July 3]. Available from: https://www.cancer.org/cancer/kidney-cancer.html
- Signoretti S, Flaifel A, Chen YB, et al. Renal cell carcinoma in the era of precision medicine: from molecular pathology to tissue-based biomarkers. J Clin Oncol. 2018;36:3553–3559.
- Viers BR, Houston Thompson R, Boorjian SA, et al. Preoperative neutrophil-lymphocyte ratio predicts death among patients with localized clear cell renal carcinoma undergoing nephrectomy. Urol Oncol. 2014;32:1277–1284.
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev cancer. 2016;16:431–446.
- Luca A, Angermayr B, Bertolini G, et al. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174–1180.
- Chao Y-N, Chiu N-C, Huang F-Y. Clinical features and prognostic factors in childhood pneumococcal meningitis. J Microbiol Immunol Infect. 2008;41:48–53.
