Abstract
Background and aim: Circulating hematopoietic stem cells (HSCs), circulating endothelial progenitor cells (EPCs) and cancer stem cells (CSCs) contribute to tumor development and progression and can predict patient outcome. The aim of this study was to investigate the frequency of circulating HSCs, EPCs and CSCs in the peripheral blood of patients with hepatocellular carcinoma (HCC) and to explore their potential prognostic significance for HCC patients.
Methods: The study included 30 HCC patients and 20 healthy controls. The HSCs and EPCs were enumerated with CD45, CD34, CD133, CD144 markers, while CSCs were enumerated with CD45, CD44, CD133 markers using flow cytometry.
Results: The mean percentages of circulating HSCs were significantly lower in HCC patients than the controls (p = .001), whereas the mean percentages of EPCs within the HSCs subpopulation were significantly higher in the HCC patients than the controls (p = .002). The absolute count of CSCs within 100,000 peripheral blood mononuclear cells was 23.5 ± 3.4 in the HCC patients. Also, the mean percentages of circulating HSCs, EPCs and the number of CSCs were significantly increased in patients with multiple hepatic focal lesions than in patients with a single hepatic focal lesion. Both circulating HSCs and EPCs showed significant positive correlation with the level of AFP and with the numbers of CSCs. In the meantime, the numbers of CSCs revealed significant direct correlation with ALT, AST and AFP levels. The one-year overall survival (OS) of the patients was 77.5%. High levels of CSCs, HSCs and EPCs at diagnosis were all associated with worse outcome for the HCC patients.
Conclusions: Significant changes in the levels of the circulating HSCs, EPCs and CSCs occur in HCC. These changes help the diagnosis and the prediction of HCC outcome, as higher levels of these cells are associated with worse OS.
Introduction
Hepatocellular carcinoma (HCC) is one of the main health problems in the world. It ranks the sixth most common type of cancer and the second main cause of malignancy-related mortality worldwide [Citation1–4]. Despite advances in curative treatment modalities for HCC including surgical resection, liver transplantation and radiofrequency ablation [Citation5–8], high recurrence rates still limit overall patients' survival [Citation9]. Therefore, understanding the fundamental biological changes in HCC responsible for its rapid growth and metastasis is essential to guide the development of new therapeutic options [Citation10–12].
The role of angiogenesis and vasculogenesis in solid tumor expansion and the importance of newly formed pathological arterial perfusion in the advancement of the malignancy had been confirmed [Citation13,Citation14]. The cell fractions coming from hematopoietic, endothelial or even the tumor cells themselves, have been implicated in originating tumor angiogenesis [Citation15,Citation16].
Previously, it was believed that the endothelial cells in the neighboring normal tissues were the original source of the recruitment of endothelial cells [Citation17]. However, further studies have demonstrated that endothelial progenitor cells (EPCs) are mobilized from the bone marrow to the peripheral blood by cytokines, and contribute to tumor neovascularization [Citation18,Citation19].
Some studies have reported that the levels of circulating hematopoietic stem cells (HSCs) and EPCs in the peripheral blood rise with advancing tumor stage [Citation20–22]. They also correlate with tumor staging, distant metastasis and recurrence after surgical treatment [Citation23,Citation24]. Thus, they could be important biomarkers for the clinical evaluation of patients with HCC, selection of treatment options and for prediction of the treatment outcome [Citation25,Citation26].
Cancer stem cells (CSCs) are a small population, constituting about 2.5% of circulating tumor cells [Citation27,Citation28]. Although the origin of CSCs is still indistinct, CSCs in HCC probably originate from de-differentiation of normal liver cells [Citation29]. Due to their stem cell-like characteristics, CSCs are capable of initiating tumor growth, maintaining self-renewal and are responsible for the distant metastasis and or tumor recurrence. They are also involved in the resistance to chemotherapy and radiotherapy [Citation29]. Therefore, elevation of circulating CSCs in the blood may be related to poor prognosis for the cancer patients [Citation30].
The use of specific surface markers for the detection of CSCs signifies their possible usefulness as molecular therapeutic targets aiming precisely towards CSCs for treatment of HCC [Citation31].
The current study was conducted to investigate the frequency of circulating HSCs, EPCs and CSCs in the peripheral blood of patients with HCC and to explore their potential prognostic significance for patients with HCC.
Patients and methods
The study included 30 patients who presented with unresectable HCC to South Egypt Cancer Institute and Assiut University Hospital. Twenty healthy volunteers of matched sex and age were also enrolled as controls. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by our Institutional Review Board, Assiut University College of Medicine (IRB no. 17300237). An informed oral consent was taken from all participants. Study subjects' privacy and confidentiality were considered during collection of the data. Participants had the right to withdraw from the study at any time and without any rational.
The diagnosis and assessment of the patients was based on clinical examination and laboratory investigations including complete blood picture (Ruby Cell Dyn fully automated blood counters, Santa Clara, CA, USA). Serum levels of albumin, total and direct bilirubin, alanine transaminase (ALT), aspartate transaminase (AST) were detected by Cobas Integra® 400 Plus, Roche Diagnostics (Mannheim, Germany). Also serum level of alpha-fetoprotein (AFP) was detected by Access 2 immunoassay system, Beckman Coulter (Brea, CA, USA). Liver dynamic (multiple phase) computed tomography or magnetic resonance imaging studies for the diagnosis and evaluation of the tumor extent inside the liver were performed for these patients. We did our investigations on naive HCC patients before the patients were treated with transarterial chemoembolization with drug-eluting beads (DEBs) loaded with 50 mg doxorubicin and 3D-conformal external beam radiotherapy (3D-CRT).
Flow cytometric detection of the circulating hematopoietic stem cell, and the circulating endothelial progenitor cells
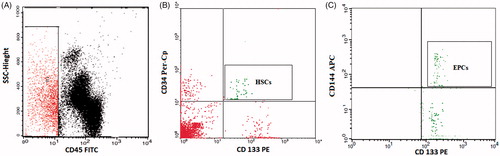
Freshly placed, flushed venous cannulas were used to obtain blood samples (). One hundred microliters of blood sample were incubated for 15 minutes with 10 µL of each of fluorescein isothiocyanate (FITC) labeled CD45 (Becton Dickinson (BD) Biosciences, San Jose, CA, USA), phycoerythrin (PE) conjugated CD133 (AC133) (MiltenyiBiotec GmbH, Bergisch Gladbach, Germany), peridinium-chlorophyll-protein (Per-CP)-conjugated CD34 (BD Biosciences, San Jose, CA, USA) and allophycocyanin (APC)-conjugated CD144 (BD Biosciences, San Jose, CA, USA). After incubation, lysis and washing of red blood cells (RBCs) were done. The cells were suspended in phosphate buffer saline (PBS) and analyzed by FACSCalibur flow cytometry with Cell Quest software (BD Biosciences, San Jose, CA, USA). Fifty thousand events were acquired, and anti-human IgG as an isotype-matched negative control was used with each sample. The circulating HSCs were defined by the phenotype of CD34+ CD133+ CD45- and quantified as a percentage of the total white blood cells population (%HSCs/WBCs). The EPCs were defined by the phenotype of CD144+ CD34+ CD133+ CD45–, and quantified as percentage from the HSCs subpopulation (% EPCs/HSCs) [Citation13,Citation32,Citation33].
Figure 1. Flow cytometric detection of the circulating hematopoietic stem cell (HSCs), and the circulating endothelial progenitor cells (EPCs). (A) The analysis gate (R1) included CD45− cells. (B) The expression of CD34 and CD133 was assessed on CD45− cells to detect hematopoietic stem cells (HSCs). (C) Then, the expression of CD144 on hematopoietic stem cells (HSCs) was assessed to detect endothelial progenitor cells (EPCs).
Flow cytometric detection of circulating cancer stem cells
Each patient’s peripheral blood mononuclear cells (PBMCs) were separated by Ficoll density gradient centrifugation (Biochrom GmbH, Berlin, Germany) (). After the centrifugation, the buffy coat layer containing mononuclear cells was transferred to a new tube to be washed by PBS. Red blood cell lysis solution was added to deplete the RBCs. They were mixed and incubated for three minutes, and then washed by PBS.
Figure 2. Flow cytometric detection of circulating cancer stem cells. (A) The CD45− cells (R1) were selected. (B) The expression of CD44 and CD133 was assessed on CD45− cells to detect the circulating cancer stem cells (CSCs).
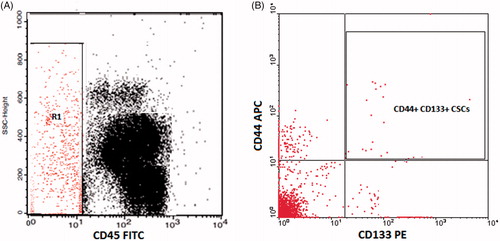
The isolated PBMCs were incubated with PE-labeled-CD133, FITC labeled CD45 and APC-labeled CD44 (BD Biosciences, San Jose, CA, USA) for 30 minutes at 4 °C. After PBS washing, about 100,000 cells were acquired by FACSCalibur flow cytometer with CellQuest software. CD45 and a side scatter histogram were used to select the CD45– cells. Subsequently, the expression of CD133 and CD44 on CD45− cells was detected. CSCs are the cells with CD44+ CD133+ CD45– [Citation34–39]. They are expressed as an absolute count of CSCs within 100,000 PBMCs.
Statistical analysis
The statistical analysis was performed using Statistical Package for Social Sciences, version 17 (IBM SPSS, Armonk, NY, USA). Results were expressed as mean ± standard error. Continuous variables and categorical variables were compared by Student’s t-test and chi-square test, respectively. Pearson's correlation coefficient was used to examine associations between the different studied variables. p Value <.05 was considered significant. Receiver operating characteristic (ROC) analysis was used to find cutoff values to designate high and low blood cell levels. Overall survival (OS) was defined as the time from diagnosis to the time of death. The Kaplan–Meier survival analysis was performed and the curves were compared by the log-rank test.
Results
Laboratory features of HCC patients and controls
The mean values of platelet count and hemoglobin concentrations were markedly reduced in HCC patients compared to the controls with highly statistically significant difference (p< .0001) (). The total WBC count was also reduced in the patients’ group, but the difference was not significant (p = .7). The effect on liver function was marked in HCC patients. The serum levels of ALT, AST, total and direct bilirubin were significantly elevated in HCC patients compared to the control group. The albumin concentration was significantly reduced in the patients’ group. The mean AFP level reached 5.8 × 103 ± 1.3 × 103 among the HCC patients.
Table 1. Laboratory features, percentages of circulating hematopoietic stem cells, endothelial progenitor cells and cancer stem cells in HCC patients and controlsTable Footnotea.
Percentages of circulating hematopoietic stem cells, endothelial progenitor cells and cancer stem cells in HCC patients and the controls
Significant changes in the percentages of circulating HSCs and the circulating EPCs were detected in the HCC patients compared to the healthy controls (). The mean percentages of circulating HSCs within the WBCs population (%HSCs/WBCs) were significantly lower in the HCC patients than the controls (p = .001), whereas the mean percentages of EPCs within the HSCs subpopulation (% EPCs/HSCs) were significantly higher in the HCC patients than the controls (p = .002). The absolute count of CSCs within 100,000 PBMCs was established to be 23.5 ± 3.4 in the HCC patients.
Laboratory features of patients groups based on hepatic focal lesions
Imaging enabled the classification of the 30 HCC patients according to the number of hepatic focal lesions into two groups: patients with a single hepatic focal lesion (group 1) and patients with multiple hepatic focal lesions (group 2) (). Comparison of the laboratory parameters between the two patients groups is presented in . The mean values of ALT, AST, AFP, total and direct bilirubin were higher in patients with multiple hepatic focal lesions (group 2) than in patients with single hepatic focal lesion (group1). However, the mean values of total WBCs count, platelet count, hemoglobin concentration and albumin concentration were lower in group 2 than in group 1. Only differences in platelet count and AFP levels were statistically significant (p = .04 and p = .01, respectively).
Table 2. Laboratory features of patients groups based on hepatic focal lesionsTable Footnotea.
Also, the mean percentages of circulating HSCs, EPCs and the number of CSCs were significantly increased in patients with multiple hepatic focal lesions (group 2) than in patients with a single hepatic focal lesion (group 1), with a p value of .046, .03 and <.0001, respectively.
Correlation between circulating HSCs, EPCs and CSCs in hepatocellular carcinoma patients and laboratory parameters
Both circulating HSCs and EPCs showed significant positive correlation with the level of AFP (r = 0.4, p = .01) and highly significant positive correlation with the numbers of CSCs (r = 0.6, p< .0001) (). The percentages of EPCs showed significant direct correlation with AST levels, but inverse correlation with platelet count (r = 0.4, p = .003 and r =–0.4, p = .005, respectively). In the meantime, the numbers of CSCs revealed significant direct correlation with ALT, AST and AFP levels (r =–0.4, p = .01, r = 0.5, p = .003 and r =–0.6, p = .001, respectively).
Table 3. Correlations between circulating HSCs, EPCs and CSCs in hepatocellular carcinoma patients and some laboratory parametersTable Footnotea.
The one-year OS of the patients was 77.5%. High levels of CSCs, HSCs and EPCs at diagnosis were all associated with worse outcome for the HCC patients. A cutoff level of 18/50,000 cells and 2/50,000 cells were used to define high and low levels of HSCs and EPCs, respectively; while the cutoff for CSCs was 18/100,000 cells. A significant difference in OS was found according to HSCs and to EPCs levels, Supplementary Figure 3 (p = .04) and Supplementary Figure 4 (p = .01), respectively. Similarly, high CSCs levels were associated with significantly worse OS, Supplementary Figure 5 (p = .002).
Discussion
Hepatocellular carcinoma is one of the main health problems and the second main cause of malignancy-related mortality worldwide [Citation40,Citation41]. The current study on circulating HSCs, EPCs and CSCs in HCC investigates the clinical significance of the evaluation of these cells for patients with HCC.
In this study, there were significant differences in the percentages of circulating HSCs and the circulating EPCs detected in the HCC patients compared to the healthy controls. The mean percentages of circulating HSCs (%HSCs/WBCs) were significantly reduced in the HCC patients, whereas the mean percentages of circulating EPCs within the HSCs subpopulation (% EPCs/HSCs) were significantly increased in the HCC patients compared to the controls. Our finding appears to be keeping with results published by Otto et al. [Citation32] who demonstrated that for a normal liver, high rate of HSCs together with low rate of EPCs is the standard. Yet, the occurrence of severe pathologic process within the liver, such as cirrhosis and particularly malignancy, inverses the relations between these cells leading to declining rates of hematopoietic cells and expanding rates of EPCs. Also, Ho et al. [Citation13] and Yang and Poon [Citation15] showed that HCC patients had significantly higher level of circulating EPCs than cirrhotic patients and healthy controls.
The mean percentages of circulating HSCs and EPCs increased significantly in patients with multiple hepatic focal lesions compared to patients with single hepatic focal lesion. These findings are consistent with other clinical studies that revealed significant increase in the levels of circulating EPCs in patients with advanced stage of HCC compared to patients with early stage of HCC [Citation13,Citation15,Citation32].
Currently, serum AFP is the most commonly used serologic marker for diagnosis of HCC [Citation42,Citation43]. Also, elevated serum levels of liver enzymes, mainly ALT and AST, are a good indicator of major hepatocellular affection [Citation44]. In this study, the percentage of circulating HSCs showed significant direct correlation with AFP level. Our observations were comparable to those reported by Ho et al. [Citation13]. On the other hand, Otto et al. found no correlation between the levels of HSCs and EPCs and the high level of serum AFP. Moreover, the percentages of HSCs and EPCs showed significant direct correlation with the number of CSCs, thus influence their relation to tumor angiogenesis and their role in detection of cancer. Also, the percentage of EPCs in this study showed significant direct relation with AST levels and inverse relation with platelet count, which supports the role of these cells in diagnosis of liver disorders. Supporting previous reports [Citation19,Citation45], these findings influence the importance of detection of HSCs and EPCs levels to help the diagnosis and point to staging of HCC, thus can help evaluation of disease and prediction of outcome.
The mean number of CSCs (expressing CD133 and CD44) within 100,000 PBMCs was established to be 23.5 ± 3.4 in our HCC patients, and showed significant increase in patients with multiple hepatic focal lesions than in patients with single hepatic focal lesion. Consistent with these results, Zhu et al. reported that tumor cells expressing both CD133 and CD44 have more stem cell properties and have the ability to initiate tumor growth with very small numbers of cells compared to CD133+ CD44− cancer cells [Citation34]. Also, Mansour et al. [Citation35] postulated that CD133+ CSCs are commonly present in HCC and the high expression of CD133 correlates with advanced tumor stage and poor prognosis for HCC patients. Furthermore, earlier studies have shown that high expression of CD133 in HCC patients is an important risk factor for tumor recurrence and OS [Citation36–38], while CD44 is independent prognostic factor for extrahepatic metastasis and short-term survival [Citation39]. In addition, over expression of both markers helps in classification of HCC patients with a high risk of tumor relapse after surgical resection [Citation45] and proposes an indicative value of CSC markers for poorly differentiated HCC [Citation46].
The numbers of CSCs in this study showed significantly positive correlation with the levels of AFP, as well as, AST and ALT. Consistent with our results, Vilchez et al. [Citation47] found that CD133+ tumor cells were independently related to high AFP levels, on multivariate analysis. Meanwhile, Mansour et al. [Citation35] reported that the correlation between CD133+ cells and the level of AFP was moderately positive but not statistically significant (r = 0.55, p = .23). The potential correlations between the CSCs and the HCC pathological serum markers as ALT, AST and AFP support the assumption that expression of CSCs markers is linked to HCC carcinogenesis [Citation48].
In addition to the clinical and pathological features, it was necessary to evaluate the correlation between levels of circulating HSCs, EPCs and CSCs and the OS of HCC patients by calculating the Kaplan–Meier survival curves. Preliminary studies performed by Guo et al. [Citation49] showed that the survival rate was higher in patients who were negative for CSCs markers. Chan et al. [Citation50] showed that immunohistochemical expression of CD133 in surgical specimens was a highly effective prognostic factor for OS in early stage HCC patients. In the current study, high levels of circulating CSCs, HSCs and EPCs expressing CD133 were associated with worse OS, as well.
Conclusions and future recommendations
Significant changes in the levels of the circulating HSCs, EPCs and CSCs occur in HCC. These changes help the diagnosis and the prediction of HCC outcome, as higher levels of these cells are associated with worse OS. Future studies should aim to explore the role of HSCs, EPCs and CSCs in early detection of HCC, monitoring of treatment and follow-up for timely recognition of relapse after treatment in those patients.
| Abbreviations | ||
| HCC | = | hepatocellular carcinoma |
| EPCs | = | endothelial progenitor cells |
| CSCs | = | cancer stem cells |
| CD | = | cluster of differentiation |
| AST | = | aspartate transferase |
| ALT | = | alanine transferase |
| AFP | = | alpha-fetoprotein |
| OS | = | overall survival |
Supplemental Material
Download MS Word (3.6 MB)Acknowledgments
The authors appreciate all the participants in this study especially the patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Hetta HF, Zahran AM, Mansor SG, et al. Frequency and Implications of myeloid-derived suppressor cells and lymphocyte subsets in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. J Med Virol. 2019;91:1319.
- Zahran AM, Abdel-Meguid MM, Ashmawy AM, et al. Frequency and implications of natural killer and natural killer T cells in hepatocellular carcinoma. Egypt J Immunol. 2018;25:45–52.
- Chaturvedi VK, Singh A, Dubey SK, et al. Molecular mechanistic insight of hepatitis B virus mediated hepatocellular carcinoma. Microb Pathog. 2019;128:184–194.
- Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70.
- T P Poon R, Ng K, Lam C-M, et al. Effectiveness of radiofrequency ablation for hepatocellular carcinomas larger than 3 cm in diameter. Arch Surg. 2004;139:281–287.
- Abd Ellah NH, Tawfeek HM, John J, et al. Nanomedicine as a future therapeutic approach for hepatitis C virus. Nanomedicine. 2019;14:1471–1491.
- Hassan EA, Ahmed EH, Nafee AM, et al. Regulatory T cells, IL10 and IL6 in HCV related hepatocellular carcinoma after transarterial chemoembolization (TACE). Egypt J Immunol. 2019;26:69–78.
- Ng KT, Poon R, Lo C-M, et al. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg. 2008;12:183–191.
- Knudsen ES, Gopal P, Singal AG. The changing landscape of hepatocellular carcinoma: etiology, genetics, and therapy. Am J Pathol. 2014;184:574–583.
- Mazzanti R, Arena U, Tassi R. Hepatocellular carcinoma: where are we? World J Exp Med. 2016;6:21–36.
- Hetta HF, Elkady A, Tohamy TA, et al. Regulatory B cells: key players in hepatocellular carcinoma progression. Gastroenterol Hepatol Open Access. 2016;5:00136.
- Ho JW, Pang RW, Lau C, et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44:836–843.
- Ding Y-t, Kumar S, Yu D-C. The role of endothelial progenitor cells in tumour vasculogenesis. Pathobiology. 2008;75:265–273.
- Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma. Anat Rec (Hoboken). 2008;291:721–734.
- Chen C-H, Li C, Tung W-C, et al. Levels and values of circulating endothelial progenitor cells, soluble angiogenic factors, and mononuclear cell apoptosis in liver cirrhosis patients. J Biomed Sci. 2012;19:66.
- Rafii S. Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest. 2000;105:17–19.
- Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201.
- Yu D, Sun X, Qiu Y, et al. Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res. 2007;13:3814–3824.
- Sieghart W, Fellner S, Reiberger T, et al. Differential role of circulating endothelial progenitor cells in cirrhotic patients with or without hepatocellular carcinoma. Dig Liver Dis. 2009;41:902–906.
- Yu D-C, Chen J, Sun X-t, et al. Mechanism of endothelial progenitor cell recruitment into neo-vessels in adjacent non-tumor tissues in hepatocellular carcinoma. BMC Cancer. 2010;10:435.
- Zhu H, Shao Q, Sun X, et al. The mobilization, recruitment and contribution of bone marrow-derived endothelial progenitor cells to the tumor neovascularization occur at an early stage and throughout the entire process of hepatocellular carcinoma growth. Oncol Rep. 2012;28:1217–1224.
- Gadelhak NA, Gadelhak SA, El Morsi DA, et al. Prognostic significance of three hepatitis markers (p53 antibodies, vascular endothelial growth factors and alpha fetoprotein) in patients with hepatocellular carcinoma. Hepatogastroenterology. 2009;56:1417–1424.
- Fürstenberger G, von Moos R, Lucas R, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94:524–531.
- Mellick A, Plummer P, Nolan D, et al. Using the transcription factor inhibitor of DNA binding 1 to selectively target endothelial progenitor cells offers novel strategies to inhibit tumor angiogenesis and growth. Cancer Res. 2010;70:7273–7282.
- Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology (Baltimore, Md). 2002;36:623–630.
- Zahran AM, Aly SS, Rayan A, et al. Survival outcomes of CD34(+)CD38(–)LSCs and their expression of CD123 in adult AML patients. Oncotarget. 2018;9:34056–34065.
- Zahran AM, Rayan A, Fakhry H, et al. Pretreatment detection of circulating and tissue CD133(+) CD44(+) cancer stem cells as a prognostic factor affecting the outcomes in Egyptian patients with colorectal cancer. Cancer Manag Res. 2019;11:1237–1248.
- Tsai KS, Yang SH, Lei YP, et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology. 2011;141:1046–1056.
- Lugli A, Iezzi G, Hostettler I, et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103:382–390.
- Pang RW, Poon RT. Cancer stem cell as a potential therapeutic target in hepatocellular carcinoma. Curr Cancer Drug Targets. 2012;112:1081–1094.
- Otto W, Krol M, Maciaszczyk M, et al. Levels and values of circulating hematopoietic and endothelial progenitor cells in patients with hepatocellular carcinoma. J Liver. 2014;03:167.
- Zahran AM, Aly SS, Altayeb HA, et al. Circulating endothelial cells and their progenitors in acute myeloid leukemia. Oncol Lett. 2016;12:1965–1970.
- Zhu Z, Hao X, Yan M, et al. Cancer stem/progenitor cells are highly enriched in CD133 + CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067–2078.
- Mansour AH, Elkhoda TR, Ayyad RS, et al. Regulation of cancer stem cell marker (CD133) by transforming growth factor beta in hepatocellular carcinoma. Int J Cancer Res. 2014;10:65–73.
- Song W, Li H, Tao K, et al. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62:1212–1218.
- Yeh CT, Kuo CJ, Lai MW, et al. CD133-positive hepatocellular carcinoma in an area endemic for hepatitis B virus infection. BMC Cancer. 2009;9:324.
- Sasaki A, Kamiyama T, Yokoo H, et al. Cytoplasmic expression of CD133 is an important risk factor for overall survival in hepatocellular carcinoma. Oncol Rep. 2010;24:537–546.
- Hirohashi K, Yamamoto T, Uenishi T, et al. CD44 and VEGF expression in extrahepatic metastasis of human hepatocellular carcinoma. Hepato-gastroenterology. 2004;51:1121–1123.
- Zahran AM, Nafady-Hego H, Mansor SG, et al. Increased frequency and FOXP3 expression of human CD8+ CD25High + lymphocytes and its relation to CD4 T regulatory cells in patients with hepatocellular carcinoma. Hum Immunol. 2019;80:510.
- Zahran AM, Zahran ZAM, El-Badawy O, et al. Prognostic impact of toll-like receptors 2 and 4 expression on monocytes in Egyptian patients with hepatocellular carcinoma. Immunol Res. 2019.
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md). 2011;53:1020–1022.
- Cancer EAFTSOTLEOFRATO. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ: Can Med Assoc J = journal de l'Association medicale canadienne. 2005;172:367–379.
- Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59:953–962.
- Liu R, Shen Y, Nan K, et al. Association between expression of cancer stem cell markers and poor differentiation of hepatocellular carcinoma: a meta-analysis (PRISMA). Medicine. 2015;94:e1306.
- Vilchez V, Zaytseva Y, Stewart R, et al. Liver cancer stem cell markers (CD133+/CD44+) are strongly associated with moderate to poorly differentiated hepatocellular carcinoma in patients undergoing transplantation.: Abstract# A387. Transplantation. 2014;98:697.
- Romano M, De Francesco F, Pirozzi G, et al. Expression of cancer stem cell biomarkers as a tool for a correct therapeutic approach to hepatocellular carcinoma. Oncoscience. 2015;2:443–456.
- Guo Z, Li LQ, Jiang JH, et al. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J Gastroenterol. 2014;20:2098–2106.
- Chan AW, Tong JH, Chan SL, et al. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology. 2014;64:935–950.
