Abstract
Background: Hepatocellular carcinoma (HCC) incidence is rising worldwide, especially due to increased detection of early-stage or small-sized tumors. Nevertheless, most of the patients are still not qualified for surgical resection at diagnosis due to the localization of the tumor, underlying liver disease or comorbidities. Stereotactic body radiation therapy (SBRT) is a radiotherapy modality which can deliver a high dose of radiation to the target tissue with a high degree of precision. It shows promise in terms of efficacy and morbidity.
Material and methods: The aim of this systematic review is to summarize current knowledge on patient-specific outcomes of SBRT for small HCC including overall survival, local control, the effect of dose escalation and the toxicity of the treatment. The systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). After a comprehensive database search, 16 studies (973 patients with 1034 lesions) were included in qualitative and quantitative analyses; 14 of them were retrospective.
Results: Average tumor diameter was 23 mm and 95% of patients were in good general condition. Median BED10 (biologically equivalent dose calculated for α/β ratio of 10 Gy) was 100 Gy (range 59.5–180 Gy). Mean weighted local control across studies was 94%, 92% and 93% at 1, 2, and 3 years, respectively. Mean weighted overall survival across studies was 90.9%, 67.5% and 73.4% at 1, 2, and 3 years, respectively. There were 171 grade 1–2 toxicities (17.5%) and 53 ≥ grade 3 toxicities (5.3%). There was no treatment-associated mortality.
Conclusion: SBRT offers high local control with overall survival that is comparable with radiofrequency ablation and surgery. Quality of findings, especially on toxicities, is decreased by incomplete reporting and retrospective designs of published studies. Therefore, there is a need for better reporting and prospective studies to univocally recommend SBRT as a definitive treatment option in the guidelines for small HCCs.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, the fifth most commonly diagnosed solid tumor and the third leading cause of cancer-related death, according to WHO (World Health Organization) [Citation1–3]. The prognosis and treatment options depend not only on the tumor stage but are also based on patients’ liver function and general condition [Citation4]. Therefore, the Barcelona Clinic Liver Cancer (BCLC) classification based on tumor extension, functional reserve of the liver and patients’ performance status is a widely accepted staging system. In HCC diagnosed in very early (BCLC stage 0) and early-stage (BCLC stage A) curative options such as surgical resection, liver transplantation, and ablation are recommended [Citation4,Citation5]. However, as little as 30% of patients diagnosed with HCC are eligible for definitive surgical treatment at diagnosis. This is usually due to performance status, localization, and stage of the disease or lack of patient’s consent to undergo major surgery. In effect, over the years, multiple minimally invasive modalities have been developed. These include thermal ablation (TA), ethanol injections (EI), transcatheter arterial chemoembolization (TACE), transarterial radioembolization (TARE), and external radiation therapy. Classically radiation therapy directed at the liver was of limited use, due to radiation-induced liver disease (RILD) [Citation6]. However, along with the development of new delivery techniques, as well as new radiotherapeutic modalities, this has changed. New treatment options include stereotactic body radiotherapy (SBRT) and charged particle therapy (CPT). The latter is especially interesting as it potentially offers high efficacy with lower toxicity, due to favorable dose distribution and higher relative biological effectiveness (RBE) of charged particles when compared to photon-based radiotherapy [Citation7]. However, availability of this method is still very limited and technologies to overcome interplay effects of organ motion are immature with particle therapy. On the other hand, SBRT is already widespread and can deliver a high dose of radiation to the target tissue with a high degree of precision. It can be an alternative option in patients when radical therapies are not feasible. SBRT has been shown to be a highly effective and well-tolerated treatment in patients with extracranial solid tumors as well as secondary liver tumors [Citation8–11]. Furthermore, SBRT is being considered as an option for HCC tumors not suitable for hepatic resection and RFA [Citation12,Citation13]. However, evidence suggests that SBRT can be highly effective in the curative treatment of HCC, especially in small tumors <5 cm [Citation14,Citation15].
For those tumors, SBRT seems to deliver ablative doses of radiation with a low risk of radiation injury. Moreover, small tumors more often have no macrovascular involvement and the risk of local recurrence after SBRT is decreased [Citation16]. For ablative treatment, this diameter was considered as the upper limit [Citation16]. Rising incidence of HCC caused by improved imaging techniques and screening of the risk-groups cause increase in small or early-stage tumors’ detection. Only 20% of these tumors are eligible for surgical treatment [Citation17]. Therefore, efficacy and morbidity of SBRT are of interest. Nevertheless, there are no systematic reviews or meta-analyses available on the early-stage HCC.
Aim
To perform a systematic review of patient-specific outcomes of SBRT for early-stage (BCLC stage 0 and A) HCC (mean diameter < 5 cm and non-advanced tumors).
Materials and methods
Study protocol
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and facilitated a PICO-styled research question (Patients, Intervention, Comparisons, Outcomes) [Citation1]. MEDLINE (PubMed) database was searched for literature published until 30 May 2019. We used the following search query: “(stereotactic OR SBRT OR SABR)) AND (HCC OR hepatocellular OR liver cell cancer OR hepatic cell)” to identify studies on SBRT for small HCC. Additionally, ClinicalTrials.gov was checked for trials on the subject.
Selection criteria and data extraction
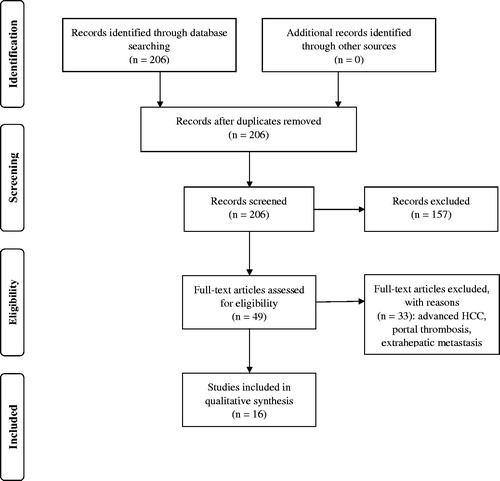
Inclusion criteria were as follows: prospective and retrospective studies, studies reporting results of SBRT on small HCC (single and multiple, median diameter <5 cm (<50 mm)), studies reporting 1, 2, or 3-year overall survival (OS) and 1, 2, or 3-year local control (LC). Exclusion criteria were as follows: duplicate studies, reviews, abstracts, case reports, conference papers, letters, and editorials, and such studies were excluded during the initial screening of titles and abstracts. The initial search returned 206 results. After screening for inclusion and exclusion criteria, 49 articles remained and were assessed to exclude studies on patients with advanced HCC, portal thrombosis, and extrahepatic metastasis. Finally, qualitative and quantitative analyses of the 16 remaining studies were performed. Inclusion criteria are presented in PICO table (). All procedures were performed by two independent researchers (M. D. and P. S.). The detailed study protocol is presented on PRISMA flowchart ().
Table 1. PICO eligibility criteria.
The following data were abstracted from original articles: authors, publication year, number of patients and lesions, sex, age, Child–Pugh score (CPS), ECOG (Eastern Cooperative Oncology Group) performance status, BCLC classification, mean tumor size, previous treatment modalities, BED (Biologically Effective Dose), number of patients with G ≥ 3 (grade ≥3) toxicity according to CTCAE (Common Terminology Criteria for Adverse Events), number of patients with RILD and number of patients with non-classic RILD), LC, and OS at all years reported. The primary endpoint of the present study was LC, while the secondary endpoints were the OS and occurrence of G3 and 4 toxicities.
Study definitions
Early-stage HCC is defined as a solitary tumor ≤5 cm in maximum diameter or as multiple nodules (≤3 total) measuring ≤3 cm in maximum diameter, without vascular invasion/extrahepatic metastasis and with CPS A or B hepatic function [Citation2]. Classic RILD was defined as anicteric hepatomegaly and ascites, or elevation of alkaline phosphatase more than twice above the upper limit of normal or baseline level and non-classic RILD was defined as an elevation in the level of transaminases or bilirubin, which was graded according to CTCAE, or a decline in liver function measured by a worsening of CPS ≥2 points [Citation18].
Data synthesis and statistical methods
Descriptive statistics were calculated with the use of means, weighted means and medians for the following: tumor size, LC, OS, follow-up, and BED10. For studies that did not report BED10 it was calculated for (α/β = 10 Gy) with the use of the following formula: (BED) = n × d (1 + d/α/β), as previously proposed (n is the number of treatment fractions and d is the dose per fraction) [Citation8,Citation19,Citation20]. Graphs were plotted to present LC and OS over the years, the relationship between LC and OS over tumor size and LC and OS over BED. All calculations and graphs were performed with MS Office Excel 16.18 (Microsoft, Redmond, WA).
Results
Study characteristics
A total of 16 articles were identified and analyzed. Characteristics of the included studies are summarized in . Of those, 14 studies were retrospective and 2 were prospective. In total there were 973 patients with 1034 lesions, what gives an average of 1.06 lesions per patient. In 4 of 16 studies (25%), only patients with small, single lesions were included; remaining studies included both single and multiple lesions. The median age of HCC patients was 67 years and 73% of patients were male. Weighted mean tumor diameter across studies was 23.15 mm. Seven studies assessed clinical stage using BCLC (395 patients). Among those, 88 patients were BCLC stage 0 (22%) and 182 were BCLC A (46%) and remaining had higher BCLC stages. Very early and early stage of HCC compromised of 270 (68%) of treated patients, in studies that used BCLC. In 7 studies, staging system used was not reported. The remaining studies used modified UICC (Union of International Cancer Control) stage or TNM Classification of Malignant Tumors 8th Edition. ECOG performance status was available in 8 of 16 studies (416 patients). Patients with ECOG 0 and 1 accounted for 94.9% of patients within studies that reported it. For GTV (gross tumor volume) imaging, all researchers used a computed tomography (CT) and magnetic resonance imaging (MRI) imaging. They used CyberKnife (35%), IMRT (intensity-modulated radiotherapy) (35%) or VMAT (Volumetric Arc Therapy) (30%) as a treatment method. In 223 patients (22.9%), SBRT was reported as the first treatment method. TACE was the most common previous treatment modality (234 patients). RFA (54 patients), surgery (31 patients), and PEI (percutaneous ethanol injection) (3 patients) were also used prior to SBRT in included studies. Median follow-up ranged from 12 to 41.7 months, with a mean of 21.8 months across studies. Treatment response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) or mRECIST (modified RECIST) criteria on multiphase CT or MRI images performed after treatment [Citation21]. One study used WHO criteria [Citation22].
Table 2. Characteristics of the included studies.
Local control
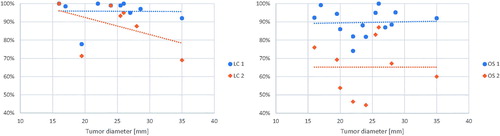
One-year LC was available in 11 of 16 studies. It ranged from 77.8% to 100% with a weighted mean of 94.1%. Local control after 2 years was available in 7 studies and ranged from 69 to 100% with a weighted mean of 92.2%. Four of 16 studies reported LC after 3 years which ranged from 91 to 97%, with a weighted mean of 93.7%. When prospective studies considered separately LC was 87.6% after 2 years [Citation23] and 96.3% at 3 years [Citation15]. Relationship between LC and tumor diameter was explored – what is shown in , left panel. Figure with data on 3-year LC presented and figures indicating study sample sizes are to be found in the Supplement.
Overall survival
Of 16 studies, 14 reported 1-year OS and it ranged from 74.1% to 100% with a weighted mean of 90.9%. Two-year OS was available in 9 studies and 4 studies presented 3-year OS. The weighted mean rates were 67.4% and 73.3% respectively. Relationships between 1- and 2-year OS and tumor diameter are presented in , right panel. Figure with data on 3-year OS presented and figures indicating study sample sizes are to be found in the Supplement.
Radiation dose
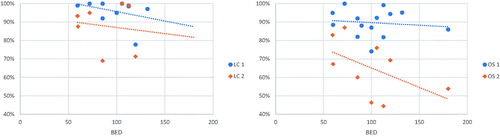
The SBRT was given in 3–6 fractions and dose per fraction ranged from 5 Gy to 20 Gy. BEDs10Gy ranged from 60 Gy to 151.2 Gy. The correlation of LC and OS over BED10Gy was plotted on graphs, as shown in , panels left and right (respectively).
Treatment-related toxicities
Treatment-related toxicity was reported in all included studies. Adverse events related to SBRT were graded according to the CTCAE in all studies. Summary of reported toxicities is presented in . All included patients completed planned treatment with no interruptions due to intolerable side effects. There were no treatment-related deaths. In 973 patients, 171 (17.6%) early G1–G2 and early 53 (5.4%) ≥G3 toxicities were reported. The most common toxicities related to SBRT treatment were G1 fatigue and nausea, with 13 patients experiencing fatigue. Classic RILD was reported in 3 (1.8%) patients [Citation24–26] and non-classic RILD in 51 (26%) patients [Citation24,Citation27]. Early toxicities ≥ G3 occurred rarely in all studies. There were no G3 toxicities in four studies [Citation26,Citation28–30]. Among reported ≥ G3 hepatic toxicity occurred in 5–6.5% of patients.
Table 3. Treatment-related toxicities.
Late toxicities related to SBRT of the liver occurred infrequently. G3 biliary strictures which required endoscopic treatment occurred in 2 (1.7%) patients 12 and 20 months after SBRT of centrally located tumors [Citation27]. Another case of biliary stricture developed in 1 (1.1%) patient 26 months after SBRT of tumor located in liver segment IV [Citation31]. Wahl et al. reported biliary toxicity in two (3.3%) SBRT-treated patients after 1 year [Citation25]. Late rib fractures occurred rarely after SBRT (3 patients). In these cases, the PTV included a fragment of the rib that has fractured.
Discussion
This review describes the efficacy and safety of SBRT in small HCC. To our knowledge, this is the first available review on this specific topic. We included 16 studies and 973 patients that showed very high LC and OS with low toxicity. Small HCC is associated with a good prognosis, and in this group of patients, SBRT may be equivalent to surgery in efficacy of the treatment and risk of complications. The rationale to perform this review is based on the rising incidence of HCC. This is mainly due to the current progress in diagnostic imaging and screening programs for HCC that caused an increase in the detection of early-stage or small-sized tumors in the liver. Those cases are considered candidates for definitive treatment. However, only 20% of these tumors can be treated via surgical resection [Citation17]. Other treatment modalities such as RFA or TACE are now the treatment of choice for unresectable lesions or in patients with poor general performance status unsuitable for surgery [Citation16]. In the group of patients with early-stage HCC (small, single lesion, unsuitable for surgery, and in good general conditions) the treatment modalities are currently not well established [Citation16,Citation32].
According to EASL (European Association for the Study of the Liver) Clinical Practice Guidelines in patients with early tumors, liver resection is considered the primary treatment method [Citation16]. It was previously reported that liver resection offers higher OS than local radiation therapies in early stages of HCC [Citation17,Citation33]. One-year OS after liver resection ranges from 93 to 100% and 2-year OS ranges from 82% to 93.5% [Citation34,Citation35]. RFA is the second recommended treatment option with previously published reports supporting its efficacy in the treatment of early HCC [Citation35,Citation36]. It is associated with 1-year OS ranging from 90% to 96% and 2-year OS ranging from 79% to 82% [Citation34,Citation37]. In our analysis, SBRT offers nearly as good 1- and 2-year LC (1-year LC 94%, 2-year LC 92%) and 1-year OS (1-year OS 91%) and comparable 2-year OS (2-year OS 67%). Such comparisons should, however, be made with caution. Both different selection criteria for patients qualified for recommended treatment modalities and varying quality of reporting in studies included in this review may potentially introduce bias, but most obviously the SBRT patients had the worse prognostic criteria. Comparing to a recently published systematic review in our study LC and OS remains stable in HCC mean diameter from 15 to 35 mm [Citation38], whereas it was proven that increasing tumor diameter is associated with lower LC and OS.
Our study shows that SBRT is associated very low levels of early and late toxicities (overall 171 G1–G2 (17.5%) and 53 ≥G3 toxicities (5.3%) reported) compared to surgery which is associated with 13% to 32% of perioperative morbidity [Citation37,Citation39]. SBRT for HCC shows a similar profile of toxicity to the widely accepted application in metastatic tumors of the liver [Citation8]. Compared to surgery patients treated with RFA have noticeably lower morbidity (9%, p = .003) [Citation37]. In the present analysis, most of the toxicities were laboratory abnormalities which are most often transient after SBRT treatment or may have been attributable to preceding TACE [Citation14,Citation40]. It is important to consider that the low incidence of early toxicities may be due to underreporting both in patients treated with SBRT and RFA.
Across the studies included in the current review, no treatment-related deaths were reported, whereas resection is associated with 1–3% surgical mortality. This should be considered preoperatively in patients with significant comorbidities or impaired liver function [Citation16]. RFA was associated with 0–15% mortality [Citation37]. Considering mortality, presented results may be considered superior to RFA and surgery.
The dose and fractionation in HCC SBRT treatment are still under investigation. Different radiation doses were administered in every study included in the analysis. The significant difference in BED10 was also observed across the studies. In included studies, delivery protocols (dose per fraction) have varied considerably from 5 to 18 Gy. There is also no consensus as to the number of fractions used in therapy; therefore, a variation of BED was visible across the studies. The total dose depends not only on liver function based on CPS but also on the restrictions of the dose delivered to the healthy liver tissue and the dose delivered to the other organs at risk (stomach, bowels, and spinal cord). Most authors did not report BED in treatment protocols. The increase in calculated BED seems not to correlate with improved LC and OS as shown in , in contrary to secondary lesions, what has been previously demonstrated [Citation8]. The benefit of dose escalation for LC and OS at 3 years which can be observed in Supplement Figure is probably illusive and possibly attributable to a low number of studies that include 3-year LC and OS. In addition, the study by Jeong et al. reported very high outcome values and, therefore, is an outlier which biases the trendline. Therefore, these results should not be interpreted. In nine studies, BED10 > 100 Gy was delivered to tumors. Studies reporting cohorts treated with lower radiation dose suggest comparable efficacy to a high dose of radiation [Citation14]. Reports on using lower dose per fraction (4–5 Gy per fraction, a total dose of 40–50 Gy) SBRT suggests good LC and safety especially in HCC located close to critical organs. Because of these differences, further studies should be conducted to clearly support the recommendation of minimum or maximum dose of SBRT for small HCC.
Limitations
There are some limitations of the current analysis to consider. First, as discussed previously [Citation7,Citation8], reporting of data is a major concern in radiation therapy studies. Presented data tend to overestimate LC rates because of low follow-up quality on a patient with progressive disease. Moreover, authors use different outcome measures (such as OS, PFS (progression-free survival), DFS (disease-free survival), LC and treatment response) assessed in different time-points what makes quantitative analyses either difficult or impossible. Furthermore, most of the studies do not report raw data that could be included in meta-analyses and rather report only calculated values. This is especially crucial when analyzing response parameters such as LC. Means that were calculated in the present analysis do not take into account how many patients were at risk (alive) at time of analysis (i.e., at 1, 2, and 3 years), as this value is often not reported by authors. This potentially may have led to the overestimation of LC, as it is in turn calculated for survivors only, making this a selection bias (survivor bias). In addition, many studies describe the dose prescription policy very poorly which complicates the correlation analysis between BED and outcomes. Moreover, SBRT modalities (VMAT, IMRT, and CyberKnife) used in the studies are different and still modified. Treatment modality was not reported in some studies which affect the analysis as well. Furthermore, a minority of included studies are prospective, and none are a randomized clinical trial. This limits the quality of analyses performed in the current study and conclusions that are drawn. Additionally, in included studies, only a quarter of the patients were treated with SBRT as a first treatment modality. Remaining patients have previously undergone other locoregional and surgical treatments. This fact indicates that the observed effect is probably a summed effect of all of the methods used in a patient. The benefit of SBRT used as a first therapy might be higher. However, despite the limitations, it is our opinion that presented analyses and results provide new insight into the efficacy of SBRT for small HCCs.
Conclusions
The application of SBRT in the first-line treatment of small HCC is still under investigation. In recommendations, it is considered as a salvage therapy in large, advanced tumor with portal thrombosis unsuitable for other local treatment modalities. Results of this review demonstrate that SBRT is a safe and effective option for small HCCs however the results are limited by the low quality of studies and heterogeneous groups of patients treated with SBRT. It offers high LC and OS, with a low toxicity related to the treatment. This provides a rationale for further studies comparing SBRT with other minimally invasive techniques as a first-line treatment for unresectable small HCCs.
Supplemental Material
Download MS Word (112.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Kamangar F, Dores G, Anderson W. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. JCO. 2006;24:2137–2150.
- Kinoshita A, Onoda H, Fushiya N, et al. Staging systems for hepatocellular carcinoma: current status and future perspectives. WJH. 2015;7:406–424.
- Llovet J, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338.
- Dawson B, Hashem S, Bujold A. Stereotactic body radiation therapy for hepatocellular carcinoma. Am Soc Clin Oncol Educ Book. 2012;118:261–264.
- Spychalski P, Kobiela J, Antoszewska M, et al. Patient specific outcomes of charged particle therapy for hepatocellular carcinoma – a systematic review and quantitative analysis. Radiother Oncol. 2019;132:127–134.
- Kobiela J, Spychalski P, Marvaso G, et al. Ablative stereotactic radiotherapy for oligometastatic colorectal cancer: systematic review. Crit Rev Oncol Hematol. 2018;129:91–101.
- Onal C, Guler O, Yildirim B. Treatment outcomes of breast cancer liver metastasis treated with stereotactic body radiotherapy. Breast. 2018; 42:150–156.
- Scorsetti M, Comito T, Clerici E, et al. Phase II trial on SBRT for unresectable liver metastases: long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat Oncol. 2018;13:234.
- Kreinbrink P, Blumenfeld P, Tolekidis G, et al. Lung stereotactic body radiation therapy (SBRT) for early-stage non-small cell lung cancer in the very elderly (≥80 years old): extremely safe and effective. J Geriatr Oncol. 2017;8:351–355.
- Rim C, Seong J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat Oncol J. 2016;34:160–167.
- Ohri N, Dawson L, Krishnan S, et al. Radiotherapy for hepatocellular carcinoma: new indications and directions for future study. J Natl Cancer Inst. 2016;108:djw133.
- Kim J, Seong J, Lee I, et al. Phase I dose-escalation study of helical intensity-modulated radiotherapy-based stereotactic body radiotherapy for hepatocellular carcinoma. Oncotarget. 2016;7:40756–40766.
- Takeda A, Sanuki N, Tsurugai Y, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122:2041–2049.
- Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015;62:617–624.
- Pan CC, Kavanagh BD, Dawson L, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:94–100.
- Carvajal C, Navarro-Martin A, Cacicedo J, et al. Stereotactic body radiotherapy for colorectal lung oligometastases: preliminary single-institution results. J BUON. 2015;20:158–165.
- Shiba S, Abe T, Shibuya K, et al. Carbon ion radiotherapy for 80 years or older patients with hepatocellular carcinoma. BMC Cancer. 2017;17:721.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
- Tirkes T, Hollar MA, Tann Dom, et al. RECIST mRECIST Choi and PERSIST criteria for tumour assessment. 2013.
- Park J, Yoon SM, Lim S, et al. Two-week schedule of hypofractionated radiotherapy as a local salvage treatment for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:1638–1642.
- Huertas A, Baumann AS, Saunier-Kubs F, et al. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115:211–216.
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34:452–459.
- Moore A, Cohen-Naftaly M, Tobar A, et al. Stereotactic body radiation therapy (SBRT) for definitive treatment and as a bridge to liver transplantation in early stage inoperable hepatocellular carcinoma. Radiat Oncol. 2017;12:4–11.
- Jeong Y, Cho B, Park JH, et al. Stereotactic body radiation therapy using respiratory-gated volumetric-modulated arc therapy technique for small hepatocellular carcinoma. Liver Cancer. 2018;4:201.
- Shiozawa K, Watanabe M, Ikehara T, et al. Comparison of percutaneous radiofrequency ablation and CyberKnife ® for initial solitary hepatocellular carcinoma: a pilot study. WJG. 2015;21:13490.
- Kimura T, Aikata H, Takahashi S, et al. Stereotactic body radiotherapy for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Hepatol Res. 2015;45:378–386.
- Hasan S, Thai N, Uemura T, et al. Hepatocellular carcinoma with child Pugh-A Cirrhosis treated with stereotactic body radiotherapy. WJGS. 2017;9:256–263.
- Yoon SM, Lim YS, Park MJ, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013;8:e79854.
- Zeng ZC, Seong J, Yoon SM, et al. Consensus on stereotactic body radiation therapy for small-sized hepatocellular carcinoma at the 7th Asia-Pacific Primary Liver Cancer Expert Meeting. Liver Cancer. 2017;6:264–274.
- Garancini M, Nespoli S, Romano F, et al. Surgical management of hepatocellular carcinoma within and beyond BCLC indications in a middle volume center. J Visc Surg. 2018;155:275–282.
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328.
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909.
- Shibata T, Isoda H, Hirokawa Y, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78.
- Rim CH, Kim CY, Yang DS, et al. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: a meta-analysis and systematic review. Radiother Oncol. 2018;129:112–122.
- Huang X, Lu S. A meta-analysis comparing the effect of anatomical resection vs. non-anatomical resection on the long-term outcomes for patients undergoing hepatic resection for hepatocellular carcinoma. Hpb. 2017;19:843–849.
- Hanazawa H, Takahashi S, Shiinoki T, et al. Clinical assessment of coiled fiducial markers as internal surrogates for hepatocellular carcinomas during gated stereotactic body radiotherapy with a real-time tumor-tracking system. Radiother Oncol. 2017;123:43–48.