Introduction
The cure rate of Hodgkin lymphoma (HL) has drastically improved over the past several decades due to advances in both chemotherapy and radiotherapy (RT). Over 90% of patients diagnosed with HL this decade will survive their disease [Citation1]. However, the therapies producing these high cure rates are not without their long-term side effects. Children participating in the St. Jude Children’s Research Hospital (Memphis, TN) SJLIFE cohort for HL developed an average of five severe, life-threatening or fatal chronic conditions by their 50th birthday [Citation2]. Recent prospective trials in both the adult and pediatric populations have aimed to reduce treatment intensity for those who respond favorably to initial chemotherapy [Citation3,Citation4].
Cardiovascular disease is of particular concern for long-term survivors of HL. In a Dutch cohort of pediatric and adult patients treated between 1965 and 1995, the cumulative incidence of cardiovascular disease 40 years after treatment was 50% [Citation5]. Likewise, among the aforementioned SJLIFE cohort, the most common chronic disease in long-term survivors of HL was cardiovascular disease. Contributing to this increased risk is the use of (1) anthracycline-containing chemotherapy and (2) mediastinal RT, both known risk factors for late cardiovascular disease [Citation5–9]. The foundational reports establishing this relationship typically consider either prescribed RT dose or mean heart RT dose. Yet, the heart is a heterogeneous organ comprised multiple substructures, each with a unique function and likely a unique predilection to RT-induced damage. Recent smaller studies have suggested that RT dose to the left ventricle (LV), left anterior descending artery (LAD) or cardiac valves may be more predictive of specific cardiovascular disease outcomes than whole-heart dose metrics [Citation10–12].
We hypothesized that the relationship between prescription RT dose and mean heart RT dose with specific cardiac substructures may differ by cardiac substructure in patients treated for HL. To evaluate this theory, we calculated the prescription dose, mean whole heart dose, and mean dose to several cardiac substructures from involved-field radiotherapy (IFRT) fields using modern treatment planning software.
Methods
Following institutional review board approval, we selected 37 patients (pediatric and adult) treated with modern involved-site RT for mediastinal HL at a single institution between 2009 and 2017. For each patient, IFRT plans were retrospectively created using historical field borders [Citation13]. Additionally, for each patient, several cardiac substructures were contoured based on previously published guidelines including the LV, right ventricle (RV), left atrium (LA), right atrium (RA), aortic valve (AV), mitral valve (MV), pulmonic valve (PV), tricuspid valve (TV), LAD, circumflex artery (CA), right coronary artery (RCA), atrioventricular node (AVN) and sinoatrial node (SAN) [Citation14,Citation15]. We created a structure comprised of the four contoured cardiac valves (AV, MV, PV and TV), and a structure comprised of the three contoured coronary arteries (LAD, CA and RCA). Mean doses to each structure (as well as to the entire heart) were calculated using MIMVista software (MIM Software Inc., Cleveland, OH, USA). The prescription dose used was the treated prescription dose for each individual patient. This dose ranged from 21 to 36 Gy as some patients treated were pediatric. Boost doses were not considered for this analysis.
Robust linear regression analysis to minimize the impact of heteroscedasticity was performed individually for each cardiac substructure with each specific substructure, once with mean heart RT dose as the independent variable and again with prescription RT dose as the independent variable. A coefficient of determination (r2) was used to determine the presence or absence of a linear relationship. A coefficient of 0.7–1.0 was considered strong, 0.5–0.69 was moderate and 0.0–0.49 was weak. Scatterplots were generated to graphically depict each relationship (Supplementary Figures). The coefficient of determination for mean heart RT dose and prescription RT dose were compared computationally via linear regression and graphically via a scatterplot. All statistical analysis was performed using StataSE software version 15 (StataCorp, College Station, TX, USA).
Results
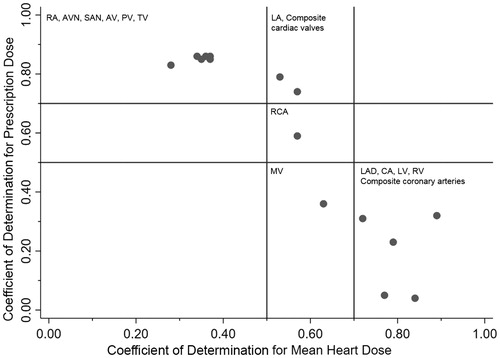
The coefficient of determination (r2) values for the relationship between each substructure and the mean heart dose or prescribed dose are shown in . The RV (r2 = 0.89), LV (r2 = 0.84), composite coronary arteries (r2 = 0.79), LAD (r2 = 0.77) and CA (r2 = 0.72) correlated strongly with mean heart dose. The RA (r2 = 0.86), PV (r2 = 0.86), AV (r2 = 0.86), AVN (r2 = 0.86), TV (r2 = 0.85), SAN (r2 = 0.83), composite cardiac valves (r2 = 0.79) and LA (r2 = 0.74) correlated strongly with prescribed RT dose. No cardiac substructure had a strong correlation with both mean heart RT dose and prescribed RT dose.
Table 1. The coefficient of determination for cardiac substructures with mean heart RT dose and prescription RT dose in patients treated for mediastinal Hodgkin lymphoma.
There was a strong correlation (r2 = 0.85) for the relationship between the cardiac substructure/mean RT dose coefficient of determination and the cardiac substructure/prescribed RT dose coefficient of determination (). We identified three groups of structures based on this relationship: (1) structures with a strong correlation with prescription dose and moderate or weak correlation with mean heart dose, including the atria and individual cardiac valves, except the MV, the SAN, AVN and composite cardiac valves; (2) structures with a strong correlation with mean heart dose and a moderate or weak correlation with prescription dose, including both ventricles, the LAD, the CA and the composite coronary arteries; and (3) structures without a strong correlation with either mean heart dose or prescription dose, including the MV and RCA.
Discussion
We show that in patients treated with IFRT for mediastinal HL, mean cardiac RT dose and prescription RT dose are correlated with a unique set of cardiac substructures. Our findings suggest that these two whole-heart dose metrics may be predictive of separate and specific late cardiac complications. Prescription RT dose is most correlated with ‘group 1’ substructures, including three of the four cardiac valves, both atria, the AV and SA nodes and the composite cardiac valves. Mean cardiac RT dose is best correlated with ‘group 2’ substructures, including both ventricles, the left-sided coronary arteries and a composite structure of all three major coronary vessels. Several substructures, those in ‘group 3’ are not strongly correlated with any dose metric, including the RCA and the MV. It is reasonable to hypothesize that, among patients treated with IFRT for HL, prescription RT dose may better correlate with late valvular disease and mean cardiac RT dose may better correlate with coronary artery disease and potentially heart failure.
Most of our knowledge on the dose-response relationship between RT dose and late cardiac complications from RT is based on whole-heart dose metrics [Citation5–9,Citation16]. Early studies evaluated the relationship between prescription RT dose and cardiac outcomes in patients treated with mediastinal radiation [Citation8,Citation9,Citation17]. Yet, as computer technology progressed, methods for estimating mean heart dose and recreating RT treatment fields on age-specific phantoms were developed that allowed for calculation of the mean heart dose in patients treated decades ago [Citation16,Citation18,Citation19]. The results were that more modern studies also came to rely on mean heart doses rather than prescription doses [Citation6,Citation7].
Attempts at conducting a meta-analysis of the dose-response relationship using all available data (including studies reporting both mean heart dose and prescription dose) struggle with how to combine these disparate techniques [Citation6]. Our data give some pause to this combination as these two dose metrics are correlated with specific cardiac substructures in different ways in patients treated with IFRT for mediastinal HL. Reports evaluating prescription dose may under-report the relationship between dose and both coronary artery disease and heart failure; reports evaluating mean heart dose may underreport the relationship between dose and valvular disease. A burgeoning evidence base suggests that dose to cardiac substructures is most closely related to specific cardiac disease than whole-heart dose metrics [Citation10–12]; we strongly urge further analysis into the relationship between specific cardiac diseases and doses to specific cardiac substructures in large cohorts of long-term cancer survivors.
There are several limitations inherent in our analysis. The IFRT plans analyzed were retrospectively created on patients treated in the modern era of HL treatment and, therefore, may not approximate directly the treatment plans deliver when IFRT was more widely used. Nevertheless, these IFRT plans were reviewed and modified by an experienced lymphoma radiation oncologist who has treated over 1,000 patients for HL. Additionally, these correlations only apply to patients treated with IFRT for mediastinal HL; we would urge other groups to assess these correlations for RT treatment techniques such as tangent fields for breast cancer or craniospinal irradiation for medulloblastoma. Also possible is that a linear relationship is not the best-fit regression model; we did not analyze any polynomial regression models.
Our study shows that, among patients treated with IFRT for HL – who represent a large proportion of the patients upon which dose–response relationships for assessing the risk of late cardiac disease have been determined – prescription RT dose and mean heart RT dose correlates with different sets of cardiac substructures. Prescription dose is best correlated with valvular structures and means heart dose is best correlated with the ventricles and coronary arteries. Our findings suggest that studies establishing a dose-response relationship that depends on whole-heart dose metrics may not report an accurate relationship for determining specific cardiac disease endpoints. We urge further investigation into the dose-response relationship between cardiac disease and doses to specific cardiac substructures.
Supplemental Material
Download PDF (1 MB)Acknowledgments
This research was made possible through the support of James E. Lockwood, Jr., Professorship.
Disclosure statement
BSH is a scientific consultant of Merck & Co., Inc. and Bristol-Myers Squibb.
References
- Correa CR, Litt HI, Hwang WT, et al. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25:3031–3037.
- Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390:2569–2582.
- Keller FG, Castellino SM, Chen L, et al. Results of the AHOD0431 trial of response adapted therapy and a salvage strategy for limited stage, classical Hodgkin lymphoma: a report from the Children’s Oncology Group. Cancer. 2018;124:3210–3219.
- Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429.
- van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017.
- Bates JE, Howell RM, Liu Q, et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the childhood cancer survivor study. J Clin Oncol. 2019;37:1090–1101.
- Haddy N, Diallo S, El-Fayech C, et al. Cardiac diseases following childhood cancer treatment: cohort study. Circulation. 2016;133:31–38.
- Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55:1145–1152.
- Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms’ Tumor Study group. J Clin Oncol. 2001;19:1926–1934.
- Cutter DJ, Schaapveld M, Darby SC, et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107:pii: djv008.
- van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017;129:2257–2265.
- Hahn E, Jiang H, Ng A, et al. Late cardiac toxicity after Mediastinal radiation therapy for Hodgkin lymphoma: contributions of coronary artery and whole heart dose-volume variables to risk prediction. Int J Radiat Oncol Biol Phys. 2017;98:1116–1123.
- Kaplan HS. Hodgkin’s disease. 2nd ed. (Commonwealth fund publications). Boston: Harvard University Press; 1980. p. 706.
- Duane F, Aznar MC, Bartlett F, et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122:416–422.
- Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998.
- Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837.
- Darby SC, Ewertz M, McGale P, et al. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med. 2013;368:987.
- Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157.