Abstract
Background: Fatigue is one of the most common and distressing long-term effects of cancer treatment. Cognitive behavioral therapy (CBT) is an evidence-based intervention for patients with severe post-cancer fatigue. CBT for fatigue is a complex intervention consisting of multiple elements like a graded activity program, regulation of the sleep-wake rhythm and reformulation of fatigue-related cognitions. The contribution of the separate elements to the positive effect of CBT on fatigue is unclear. The main objective of this pragmatic crossover trial was comparing the efficacy of graded activity with the other elements of CBT in reducing post-cancer fatigue.
Material and methods: Severely fatigued cancer survivors were randomized to (i) graded activity followed by the other elements of CBT after crossover (n = 41), or (ii) the two components in reverse order (n = 48). Fatigue severity was measured at baseline, before crossover and after CBT (Checklist Individual Strength (CIS), Fatigue Severity subscale). Differences in effects on fatigue were examined with a linear regression analysis. Objective physical activity, perceived activity and self-efficacy were explored as mediators of the effect of graded activity.
Results: Before crossover, the reduction in fatigue was significantly larger after graded activity than after the other elements (β = 4.75, 95% confidence interval (95% CI) = −9.19; −0.32). An increase in perceived activity mediated this effect (β = −4.17, 95% CI = −7.37; −1.37).
Conclusions: Graded activity is an important component of CBT for post-cancer fatigue as it resulted in a larger reduction in fatigue compared with the other elements, mediated by an increased level of perceived activity. Results indicated that the other elements of CBT are of added value in reducing fatigue.
Background
Fatigue is a common long-term effect of cancer treatment with a negative impact on quality of life [Citation1]. Our research group has developed cognitive behavioral therapy (CBT), specifically for severe fatigue in cancer survivors. The efficacy of the intervention in reducing fatigue was shown in three randomized controlled trials (RCTs), with clinically significant improvement in the majority of patients [Citation2–4]. CBT for post-cancer fatigue consists of six treatment modules that each correspond with a factor that can perpetuate post-cancer fatigue. One of these fatigue-perpetuating factors can be a fluctuating or low (physical) activity pattern, which is addressed in a graded activity program that is followed by all patients. Based on the literature, five other fatigue-perpetuating factors can be a deregulated sleep-wake rhythm, difficulties to cope with cancer and cancer treatment, high fear of cancer recurrence, dysfunctional cognitions regarding fatigue and low social support and negative interactions [Citation1]. The effect of the graded activity component compared to the other elements of CBT on fatigue severity has not been examined yet.
A deeper understanding of the mechanisms of change of CBT for post-cancer fatigue can be obtained by conducting mediation analyses [Citation5]. A previous study of our research group showed that an increase in objective physical activity did not mediate the effect of CBT on severe post-cancer fatigue [Citation6]. This was also found in CBT for chronic fatigue in other patient populations (e.g., chronic fatigue syndrome and Diabetes type 1) [Citation7–9]. In these populations, the effect of CBT on fatigue severity was mediated by an increase in the level of perceived activity and sense of control with respect to fatigue [Citation7,Citation8,Citation10].
It is unclear if these fatigue-related beliefs also mediate the effect of CBT on severe post-cancer fatigue. We conducted a pragmatic clinical crossover trial to examine the effect of the graded activity component of CBT in reducing fatigue severity compared to the other elements of CBT. We expected a higher reduction in fatigue severity after graded activity compared to the other elements. Changes in objective physical activity, perceived activity and self-efficacy were explored as potential mediators of this effect.
Method
This study was reported according to the criteria of the consolidated standards of reporting trials (CONSORT) statement [Citation11].
Study design
A pragmatic clinical crossover trial was conducted as part of routine clinical care. Data were collected at a treatment center for chronic fatigue, located in Nijmegen, The Netherlands. The study was approved by the Arnhem-Nijmegen Medical Research Ethics Committee and registered in the Dutch Trial Registry (no. NTR1063).
Participants and procedure
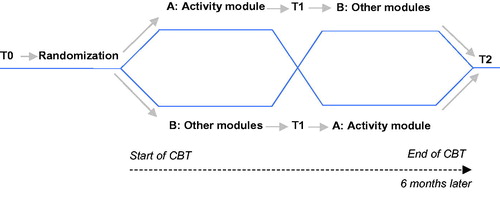
All patients referred for face-to-face CBT for post-cancer fatigue underwent a standard diagnostic procedure. Inclusion criteria were (1) ≥18 years old; (2) severe fatigue (Fatigue Severity subscale, Checklist Individual Strength (CIS-Fatigue) ≥35) [Citation12]; (3) cancer-related onset of fatigue and (4) free of cancer and completion of cancer treatment (assessed by a medical doctor). Exclusion criteria were (a) a medical condition or psychiatric disorder that explained the fatigue and (b) current psychological treatment. Patients who fulfilled the eligibility criteria received verbal and written information about the study from their therapist. Participants gave written informed consent and were randomized to either graded activity followed by the other elements of CBT (Group AB) or a condition with the same components in reverse order (Group BA). Assessments were administered at the treatment center at baseline (T0), after the first component (T1, before crossover) and both components (T2, post-CBT) ().
Randomization
An independent statistician composed a computer-generated consecutively numbered list with allocated conditions (Group AB or BA) using block-randomization (block sizes of 4). An independent test-assistant put condition labels in sealed envelopes with the consecutive numbers. Each newly included participant was randomized when the research assistant opened the envelope with the next number in presence of the patient. The nature of the study made it impossible to blind therapists for the allocated condition. However, participants were unaware of the specific content of the treatment modules until after crossover.
Intervention
The total intervention was intended to consist of 12–14 one-hour sessions in a period of six months [Citation3]. All therapists had followed a post-doctoral training in CBT and a 4-day training in providing CBT for post-cancer fatigue and participated in wwo-weekly group supervisions led by an experienced clinical psychologist (HK or TB).
Patients started CBT with formulating treatment goals. The rationale of CBT was that fatigue had been triggered by cancer and treatment of cancer. After completion of curative cancer treatment, these factors were no longer actively involved and other (psychosocial and behavioral) factors came into play. Six factors that could perpetuate fatigue were addressed in corresponding treatment modules (component A and B) [Citation3].
Component A: graded activity
All patients followed a graded activity program. An actometer was worn for 12 consecutive days and nights and was used to identify:
A fluctuating activity pattern: short periods of high activity alternated with longer periods of rest [Citation13]. Patients first learned to spread their activities more evenly throughout the day and week. Then they increased their physical activity level using a time-contingent approach. They chose a simple low-intensity activity that they could perform twice a day (like walking or cycling). Patients were instructed to start with a number of minutes that was easily attainable for them (e.g., 5 min, twice a day) and to increase this level step by step. They increased the duration of this activity with 1 min a day, progressing toward maximally 60 min, twice a day [Citation13].
A low activity pattern: these patients immediately started with graded activity. They were also instructed to choose a simple low-intensity activity and to start with an easily attainable number of minutes. They increased the duration of this activity with 1 min a day for 3–5 times a day, progressing toward maximally 60 min (twice a day) [Citation13].
When a patient had built up until at least 30 min of walking or cycling and felt like he/she could do more, the activity program was completed. The program was always ended when patients had reached 60 min of walking or cycling, twice a day, even if they did not feel like they could do more at that point [Citation3].
Component B: other modules of CBT
CBT also included five elements in which the other fatigue-perpetuating factors were addressed. The intervention was personalized: patients only followed one to maximally five of the other elements that were applicable to them. The following instruments were used at baseline to determine which fatigue-perpetuating factors were indicated for each patient:
Deregulated sleep-wake rhythm (sleep-wake diary of bed- and wake-up times during 2 weeks).
Difficulties to cope with cancer and cancer treatment (Intrusion and Avoidance subscales, Impact of Event Scale [Citation14,Citation15]).
High fear of cancer recurrence (Cancer Acceptance Scale) [Citation16].
Dysfunctional fatigue-related cognitions (Self-Efficacy Scale [Citation17], Fatigue Catastrophizing Scale [Citation18], Causal Attribution List) [Citation19].
Low social support and negative interactions (Social Support List-Discrepancies and Negative interaction subscales [Citation20,Citation21]).
Protocol fidelity
After each session, therapists registered the treatment modules that were discussed. This registry and medical files were used to assess adherence to the research protocol, based on the following criteria to prevent carryover effects: (i) T1 was administered before crossover and (ii) no elements from the other component were introduced before this transition. Patients were excluded from the analyses if these criteria were not met.
Primary outcome
Fatigue severity, measured with the CIS-fatigue (8 items, scored on a 7-point Likert scale, range 8–56). A higher score indicates a higher level of fatigue. The CIS-fatigue has a validated cutoff score for severe fatigue in cancer survivors of ≥35 [Citation12] and is a valid, reliable measure. The CIS-fatigue is sensitive to detect change in cancer patients [Citation3,Citation4,Citation22,Citation23,Citation24].
Potential mediators
Objective physical activity, measured after the graded activity program using an actometer (©Actilog version 3.0), worn for 12 consecutive days and nights. The average number of accelerations per 5-min periods over 12 d reflected a general physical activity score [Citation13]. Higher scores indicated higher activity levels. The actometer is a reliable and valid instrument [Citation13].
Perceived activity was measured with the subscale Activity of the CIS Activity: 7-point Likert scale, 3 items, range 3–21). Higher scores indicate that patients perceive their levels of activity as lower. The CIS activity has high internal consistency and test–retest reliability [Citation12].
Self-efficacy regarding fatigue was measured with the Self-Efficacy Scale (7 items, 4-point Likert scale, range 7–28) [Citation25]. Higher scores indicate a higher sense of control regarding fatigue. The SES has sufficient internal consistency [Citation17,Citation25].
Statistical analysis
Sample size calculation
To detect a clinically relevant difference in fatigue severity of 8 points (SD 14.9) between both conditions with a two-sided 5% significance level and a power of 80%, a sample size of 56 patients per condition was necessary [Citation3]. To calculate the sample size needed for an analysis of covariance (ANCOVA), we used the formula of Borm et al. [Citation26] based on the correlation between the CIS-fatigue at baseline and post-CBT from the previous RCT (r = 0.46) [Citation3]. We multiplied the sample size of 98 with the formula (1 − 0.462), resulting in a sample size of 44 patients per treatment arm [Citation26]. With an anticipated attrition rate of 20%, our accrual target was 55 participants per condition.
Fatigue severity over time
Comparability of baseline patient characteristics of both conditions was checked using independent samples t-tests and chi-squared tests. Independent samples t-tests were also conducted to examine differences in baseline characteristics between patients with and without missing data to determine if missing data were selective. The two conditions were not statistically compared at post-CBT assessment because of assumed carry-over effects after crossover. However, within group changes in fatigue severity over time were studied for each condition separately using longitudinal repeated measures ANCOVA. The reliable change index (RCI), calculated with the Jacobson Truax method [Citation27], was used to estimate the proportion of patients with meaningful changes in fatigue at T1 and T2. Clinically significant improvement was defined as a RCI of at least 1.96 [Citation27] and a fatigue level within the normal range (CIS-fatigue <35) [Citation12].
Overall effect of graded activity
We used a multivariate linear regression analysis to evaluate differences in effects between graded activity and other elements of CBT in reducing post-cancer fatigue before crossover. Condition (Group AB or BA) was regressed on fatigue severity assessed at T1, adjusted for baseline fatigue scores.
Potential mediators of the effect of graded activity
Residual change scores of the mediator variables (objective physical activity, perceived activity and self-efficacy at T1) and the dependent variable (fatigue severity at T1) were calculated. These scores were obtained by regressing T1-scores onto baseline scores and represent the amount of change in the variables independent of baseline scores.
A series of linear regression analyses were conducted on the residual change scores following a product of coefficient method (2012) [Citation28]. We calculated the effect of: (i) graded activity on fatigue severity (total effect: c-path); (ii) graded activity component on the potential mediator (a-path), and (iii) the association between changes in the potential mediator and changes in fatigue severity (b-path). The final regression model showed estimates for the b-coefficients and the direct association (c’-path). The product of coefficients (a × b) represented the relative strength of the mediation effect.
Subsequently, 95% bias-corrected accelerated CI around the mediated and direct effects were calculated with a bootstrapping method (5000 bootstrap resamples) using a SPSS macro [Citation29]. Statistical significance was tested with a nonparametric bootstrap approach (5000 data sets), created by resampling subjects from the original dataset. This approach is recommended to increase power in case of small sample sizes. The null hypothesis was rejected if the 95% CI did not include zero [Citation29]. All analyses were conducted with SPSS 24 and based on complete cases because of the focus on underlying mediating associations [Citation30].
Results
Sample characteristics
A total of 141 patients were randomized into condition AB (n = 70) and BA (n = 71) between May 2007 and September 2016 (). The research protocol was violated in 17 patients (12%), mostly because T1 was administered after crossover. All cases with protocol violations were excluded from the analyses. The T1 assessment of 35 of 124 remaining patients was missing (28%), and the T2 assessment of three additional patients (3%). In the majority of missing assessments (22 out of 35/63%), patients had stopped CBT prematurely. Most common reasons for drop-out were unmet expectations about CBT (n = 5) and cancer recurrence (n = 4). To prevent the study from being underpowered, patient recruitment was continued until reaching the required number of complete cases at T1.
Figure 2. Flow-chart of patient participation. Group AB = graded activity followed by the other elements/condition BA = other elements followed by graded activity. T0: baseline assessment; T1: assessment before crossover; T2: assessment at end of therapy.
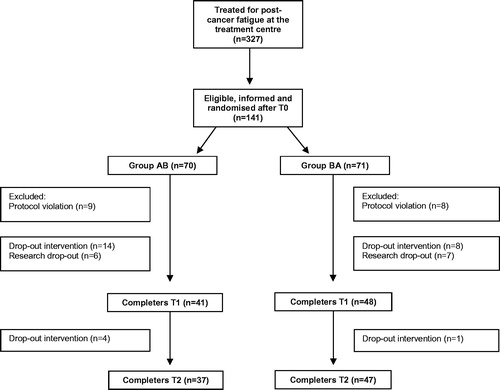
The mean number of sessions before cross-over was six for graded activity (standard deviation (SD)=2; range 4–9) and the other elements of CBT (SD = 3; range 2–13). After cross-over, the mean number of sessions was five for graded activity after the other elements (SD = 3; range 3–10) and six for the other elements after graded activity (SD = 2; range 1–9). Patients in Group AB and BA did not differ on baseline characteristics ().
Table 1. Baseline characteristics of completers of T1.
Scores on all outcomes at T0 and T1 are shown in . Baseline scores on fatigue severity and potential mediators did not differ significantly between completers and dropouts. However, completers were younger (mean age 45.7, SD = 11.9) than dropouts (mean age = 51.2, SD = 12.4). Besides, completers were more often highly educated (38% low, 7% middle, 55% high) compared with dropouts (53% low, 14% middle, 33% high). The elements that were addressed in component B are shown in Supplementary appendix Table S1.
Table 2. Scores on all outcomes in the graded activity group (A) and the group that received the other CBT elements (B) at T0 and T1.
Change in fatigue severity over time
Changes in fatigue severity over time in both conditions are visualized in Supplementary appendix Figure S1. Longitudinal analyses of both conditions separately showed that fatigue reduced significantly between T0, T1 and T2 in condition AB (p<.001) and in condition BA (p<.001). Before crossover, proportions of patients with clinically significant improvement were 51% after graded activity and 46% after the other elements of CBT (T1). These proportions were respectively 64% and 63% after CBT (T2).
Overall effect of graded activity
We found a significant effect of the graded activity intervention on fatigue severity compared with other CBT components (β = −4.99, 95% confidence interval (95%CI) = −9.40; −0.58, c-path).
Potential mediators of the effect of graded activity
Regarding the effect of graded activity on the potential mediator (a-path), there was a significantly larger increase in perceived activity (β = −2.72, 95%CI = −4.52; −0.92) and objective physical activity (β = 11.62, 95%CI = 4.98; 18.26) after graded activity than after the other elements of CBT. Regarding the association between the potential mediator and fatigue severity (b-path), changes in perceived activity (β = 1.53, 95%CI = 1.13; 1.94) and self-efficacy (β = −1.82, 95%CI = −2.52; −1.12) were significant and associated with changes in fatigue severity, while changes in objective physical activity were not. Only perceived activity significantly mediated the effect of graded activity on fatigue severity (a × b = −4.17, 95%CI= −7.37; −1.37). No significant mediation effects were found for objective physical activity and self-efficacy ().
Table 3. The total (c-path) and direct effect of graded activity on fatigue severity (c’-path), the effect of graded activity on the potential mediators (a-path), the effect of the potential mediator on fatigue severity (b-path) and the mediation effect (a × b).
Discussion
This was the first study that allowed for a direct comparison of different components of CBT for severe post-cancer fatigue. We found a larger reduction in fatigue severity after the graded activity component than after the other modules in CBT. This effect was mediated by an increase in a patient’s perceived level of activity, but not by an increase in objectively assessed physical activity.
The significant difference in fatigue severity in favor of the graded activity component implies that graded activity is an important component in CBT. However, this does not mean that graded activity alone is sufficient to manage severe post-cancer fatigue. The finding that a further reduction of fatigue was obtained after completion of all modules indicates that the other modules in CBT are of added value. When the other relevant modules were provided after crossover, the percentage of patients with clinically significant improvement in fatigue increased with 13%. Furthermore, a substantial proportion of patients (46%) showed clinically significant improvement in fatigue after the other elements (without graded activity, before crossover).
The further reduction of fatigue severity after the other modules is relevant. A long-term follow-up study showed that fatigue levels at end of CBT predicted fatigue severity up to 14 years after therapy. The lower levels of fatigue were directly after therapy, the less likely patients were to show relapse of severe fatigue in the long-term [Citation31]. This suggests that a larger reduction in fatigue at end of CBT could be beneficial for a patient’s long-term prognosis.
Perceived activity mediated the effect of graded activity on fatigue severity. This was also found in studies on mediators of the effect of CBT on fatigue severity in patients with chronic fatigue syndrome, multiple sclerosis and type 1 diabetes [Citation7,Citation8,Citation10,Citation32]. Apparently, doing more and increasing objectively assessed activity levels is not enough. To obtain a reduction in fatigue, a change in cognitions seems crucial.
In contrast to our findings, self-efficacy was identified as mediator of the effect of interventions for chronic fatigue (e.g., exercise and CBT) in different patient populations [Citation7,Citation8,Citation33,Citation34]. We found that an increase in self-efficacy was associated with a reduction in fatigue in both conditions, but not specifically with graded activity. In contrast to perceived levels of activity, an increase in self-efficacy seems to be a generic rather than an intervention-specific component in reducing fatigue. In future research, it should be explored which mediators are specific to certain elements of CBT (like perceived activity for graded activity) or more likely to be generic (like self-efficacy). In this context, other mediators need to be examined as well. For instance, depressive symptoms partly mediated the effect of CBT on fatigue severity in patients with type 1 diabetes [Citation7]. A change in depressive symptoms may also contribute to the effect of CBT for cancer-related fatigue. This should be explored in future work.
Strengths of this study include its pragmatic design and the ability to unravel the effect of graded activity compared to the other elements of CBT for post-cancer fatigue. This study also has its limitations, with the high attrition rate as the most critical issue. Drop-outs concerned a group of patients who were older and lower educated than study completers. This makes it unsure if our study sample is representative for the population of cancer survivors referred for treatment of fatigue. Reasons for nonparticipation and characteristics of the group of patients who did not want to participate in the study were largely unknown. It is unlikely that data were missing at random and we could not impute missing values. Nevertheless, the intervention effect in our study was comparable to two previous RCT’s testing the same intervention that had low treatment dropout rates (8–13%) [Citation3,Citation4]. This gives us reason to expect that the missing data did not bias our results.
We do not know if patients may have followed another physical activity program during CBT. However, it is very unlikely that a substantial number of patients in both conditions did this. Therapists actively discouraged patients to follow another physical activity program during CBT to ensure a gradual increase of activity. Our findings also showed a larger increase of objective physical activity after graded activity compared to the other modules. This suggests a limited tendency to increase physical activity in another program. Furthermore, the essence of graded activity in CBT is not only increasing levels of activity, but also changing a deregulated activity pattern and/or challenging activity-related cognitions. Therefore, even if some patients followed another physical activity program during CBT, we expect that this will have limited effect on our findings.
Moreover, our outcome was fatigue severity but fatigue is not a unidimensional symptom [Citation35]. Future studies need to distinguish the effect of CBT on different aspects of fatigue like physical versus mental fatigue. Besides, the small sample size of our study prevented us from analyzing each of the five other modules from our second component separately. This is essential for a better understanding of working ingredients of CBT. Another recommendation for future studies is analyzing subgroups of patients with different levels of physical activity at baseline to see if the effect of CBT differs between these subgroups. It would also relevant to explore if the number of sessions that a patient received on graded activity (and other modules) influence the intervention effect.
We cannot determine if patients were unaware about the content of the second component until after crossover. It is inherent to this type of research that it is difficult to realize such unawareness. In retrospect, audiotaped session recordings would have been a more reliable method to check for protocol violations of therapists. However, in that case patients could still be informed about the second component in other ways, like information on the internet.
In conclusion, graded activity is an important component of CBT for fatigue in cancer survivors. This component resulted in a larger reduction in fatigue than the other elements of CBT, mediated by an increased level of perceived activity. Results indicated that the other elements of CBT are of added value in reducing fatigue severity. Further research is needed to explore working elements of CBT for post-cancer fatigue in further detail.
Supplemental Material
Download PDF (14.7 KB)Acknowledgments
The authors thank all patients who participated in the study; Lianne Vermeeren and Liesbeth Nieboer for their assistance with the data collection and Ellen Klerks-Peters, Susanne Steur, Thea Berends, Hein Voskamp†, Dennis Marcelissen, Linde Nijhof, and José de la Fonteijne for delivering the therapy. Thea Berends was also one of the supervisors of the therapists.
Disclosure statement
The authors report no conflicts of interest.
References
- Abrahams H, Gielissen M, Verhagen C, et al. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: a systematic review. Clin Psychol Rev. 2018;63:1–11.
- Prinsen H, Bleijenberg G, Heijmen L, et al. The role of physical activity and physical fitness in postcancer fatigue: a randomized controlled trial. Support Care Cancer. 2013;21(8):2279–2288.
- Gielissen MF, Verhagen S, Witjes F, et al. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Oncol. 2006;24(30):4882–4887.
- Abrahams HJ, Gielissen MF, Donders RR, et al. The efficacy of Internet‐based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: a randomized controlled trial. Cancer. 2017;123(19):3825–3834.
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27.
- Gielissen MF, Wiborg JF, Verhagen CA, et al. Examining the role of physical activity in reducing postcancer fatigue. Support Care Cancer. 2012;20(7):1441–1447.
- Menting J, Tack CJ, Donders R, et al. Potential mechanisms involved in the effect of cognitive behavioral therapy on fatigue severity in Type 1 diabetes. J Consult Clin Psychol. 2018;86(4):330.
- Heins MJ, Knoop H, Burk WJ, et al. The process of cognitive behaviour therapy for chronic fatigue syndrome: which changes in perpetuating cognitions and behaviour are related to a reduction in fatigue? J Psychosom Res. 2013;75(3):235–241.
- Wiborg JF, Knoop H, Stulemeijer M, et al. How does cognitive behaviour therapy reduce fatigue in patients with chronic fatigue syndrome? The role of physical activity. Psychol Med. 2010;40(8):1281–1287.
- Knoop H, Van Kessel K, Moss-Morris R. Which cognitions and behaviours mediate the positive effect of cognitive behavioural therapy on fatigue in patients with multiple sclerosis? Psychol Med. 2012;42(1):205–213.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18.
- Worm-Smeitink M, Gielissen M, Bloot L, et al. The assessment of fatigue: psychometric qualities and norms for the checklist individual strength. J Psychosom Res. 2017;98:40–46.
- van der Werf SP, Prins JB, Vercoulen JH, et al. Identifying physical activity patterns in chronic fatigue syndrome using actigraphic assessment. J Psychosom Res. 2000;49(5):373–379.
- Kleber R. Schokverwerkingslijst informatie. Psychologie. 1985;40:164–168.
- van der Ploeg E, Mooren T, Kleber RJ, et al. Construct validation of the Dutch version of the impact of event scale. Psychol Assess. 2004;16(1):16.
- Gielissen M, Verhagen C, Bleijenberg G. Cognitive behaviour therapy for fatigued cancer survivors: long-term follow-up. Br J Cancer. 2007;97(5):612.
- Prins JB, Bleijenberg G, Bazelmans E, et al. Cognitive behaviour therapy for chronic fatigue syndrome: a multicentre randomised controlled trial. Lancet. 2001;357(9259):841–847.
- Jacobsen PB, Andrykowski MA, Thors CL. Relationship of catastrophizing to fatigue among women receiving treatment for breast cancer. J Consult Clin Psychol. 2004;72(2):355.
- Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: a cross-sectional study. Ann Oncol. 2002;13(4):589–598.
- Van Sonderen E. The measurement of social support with the Social Support List-Interactions (SSL-I) and the Social Support List-Discrepancies (SSL-D). Dutch Manual. Groningen, The Netherlands: Northern Center for Healthcare Research; 1993.
- Van Sonderen E, Ormel E. Measuring aspects of social support and their relationship with well-being: A study into the quality of the SSL-I and the SSL-D. Gedrag en Gezondheid. 1997;25:190–200.
- Prinsen H, van Dijk JP, Zwarts MJ, et al. The role of central and peripheral muscle fatigue in postcancer fatigue: a randomized controlled trial. J Pain Symptom Manage. 2015;49(2):173–182.
- van den Berg SW, Gielissen MF, Custers JA, et al. BREATH: web-based self-management for psychological adjustment after primary breast cancer—results of a multicenter randomized controlled trial. J Clin Oncol. 2015;33(25):2763–2771.
- van der Lee ML, Garssen B. Mindfulness‐based cognitive therapy reduces chronic cancer‐related fatigue: a treatment study. Psycho Oncol. 2012;21(3):264–272.
- de Vree B, van der Werf S, Prins J. Measurement instruments for chronic fatigue. Gedragstherapie. 2002;35–157–164.
- Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60(12):1234–1238.
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12.
- MacKinnon D. Introduction to statistical mediation analysis. Abingdon: Routledge; 2012.
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731.
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213.
- Van Gessel LD, Abrahams HJ, Prinsen H, et al. Are the effects of cognitive behavior therapy for severe fatigue in cancer survivors sustained up to 14 years after therapy? J Cancer Surviv. 2018:12:519–527.
- Chalder T, Goldsmith KA, White PD, et al. Rehabilitative therapies for chronic fatigue syndrome: a secondary mediation analysis of the PACE trial. Lancet Psychiatry. 2015;2(2):141–152.
- Rogers LQ, Vicari S, Trammell R, et al. Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Med Sci Sports Exerc. 2014;46(6):1077.
- Buffart L, Ros W, Chinapaw M, et al. Mediators of physical exercise for improvement in cancer survivors’ quality of life. Psycho Oncol. 2014;23(3):330–338.
- Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res. 2004;56(2):157–170.