Abstract
Background: Oropharyngeal squamous cell carcinomas (OPSCC) are rising rapidly in incidence due to Human Papillomavirus (HPV) and/or tobacco smoking. Prognosis is better for patients with HPV-positive disease, but may also be influenced by tobacco smoking and other factors. There is a need to individualize treatment to minimize morbidity and improve prognosis. Patient-derived xenografts (PDX) is an emerging pre-clinical research model that may more accurately reflect the human disease, and is an attractive platform to study disease biology and develop treatments and biomarkers. In this study we describe the establishment of PDX models, compare PDX tumors to the human original, and assess the suitability of this model for radiotherapy research and biomarker development.
Material and methods: Tumor biopsies from 34 patients with previously untreated OPSCC were implanted in immunodeficient mice, giving rise to 12 squamous cell carcinoma PDX models (7 HPV+, 5 HPV-). Primary and PDX tumors were characterized extensively, examining histology, immunohistochemistry, cancer gene sequencing and gene expression analysis. Radiosensitivity was assessed in vivo in a growth delay assay.
Results: Established PDX models maintained histological and immunohistochemical characteristics as well as HPV-status of the primary tumor. Important cancer driver gene mutations, e.g., in TP53, PIK3CA and others, were preserved. Gene expression related to cancer stem cell markers and gene expression subtype were preserved, while gene expression related to hypoxia and immune response differed. Radiosensitivity studies showed high concordance with clinical observations.
Conclusion: PDX from OPSCC preserves important molecular characteristics of the human primary tumor. Radiosensitivity were in accordance with clinically observed treatment response. The PDX model is a clinically relevant surrogate model of head and neck cancer. Perspectives include increased understanding of disease biology, which could lead to development of novel treatments and biomarkers.
Introduction
Oropharyngeal squamous cell carcinoma (OPSCC) is rising rapidly in incidence due to the carcinogenic effects of tobacco smoking or Human papillomavirus (HPV), and in some patients perhaps a combination of the two [Citation1,Citation2]. Although patients with HPV-positive (HPV+) OPSCC have a favorable prognosis after primary radiotherapy, and could potentially be spared side-effects if treated less intensively, individualization of treatment based on HPV-status alone remains controversial [Citation3–5]. Specifically, patients with HPV + OPSCC and a significant smoking history appear to have intermediate outcome [Citation4,Citation6]. Thus, there is a need for further insights into disease biology and development of clinically relevant biomarkers and novel treatments.
Patient-derived xenografts (PDX), where clinical tumor samples are implanted directly in mice, are being increasingly used as high fidelity pre-clinical research models of human cancer. Compared with cancer cell lines or cell line xenografts, PDX models may more closely resemble their human original tumors with regards to molecular characteristics and treatment response [Citation7–9], and may retain inter- and intra-tumor heterogeneity that is typical of human cancer [Citation10]. This is why PDX could be an attractive model to explore disease biology and radiotherapy response, and develop biomarkers and treatments. Still, it is needed to confirm that established PDX models preserve the characteristics of the original human tumor, and are suitable for further research purposes.
Thus, the aims of this study were to create a range of PDX models from OPSCC, compare PDX tumors with the human original, and explore their applicability in radiotherapy research.
Material and methods
Patients and tumor samples
Patients with previously untreated OPSCC, who consented to donation of excess tumor tissue, were eligible for this prospective study. We included consecutive patients who underwent examination under anesthesia with diagnostic biopsies or tonsillectomy on suspicion of OPSCC.
Before xenotransplantation, diagnosis was confirmed using frozen-section histology. Fresh tumor tissue of approximately 0.5 cm3 was transferred to a 2 mL Eppendorf tube containing 0.5 mL transport medium and kept on ice until xenotransplantation. Transport medium consisted of DMEM (Dulbecco’s Modified Eagle Medium, Gibco) supplemented with FBS (Fetal Bovine Serum, Gibco), amphotericin B and penicillin/streptomycin.
Creation of patient-derived xenografts
For each patient sample, 3–4 female mice (NSG, Jackson Laboratories, or CIEA NOG, Taconic) were inoculated. Mice were anesthetized with ketamine/xylazin i.p. The tumor specimen was finely minced in the Eppendorf tube using fine scissors, and Matrigel Matrix HC (BD Biosciences) was added to the mixture, which was aspirated to a syringe. Mice were inoculated subcutaneously in bilateral flanks using a needle.
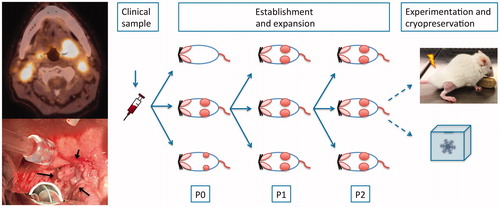
Mice were observed for tumor growth for at least 3 months. If successful xenografting occurred, the mouse was sacrificed by cervical dislocation once the largest tumor reached a volume of 1 cm3, or total tumor volume exceeded 10% of bodyweight. Tumors were harvested and transplanted to a new generation of mice using similar procedure. Generally, we selected the largest and fastest growing tumors for further transplantation. This was repeated for 3-5 generations, creating cohorts of similar tumors. First generation of PDX tumors were termed P0, with subsequent generations named P1, P2, etc (). Tumor tissue was collected at each passage for later analysis. Also, tumor tissue was cryopreserved for later revival of PDX models. For this purpose, tumor tissue was minced in Transport medium supplemented with dimethyl sulfoxide (DMSO), and the sample was frozen in a controlled-rate freezing container to −180° C.
Figure 1. Establishment of PDX models. Left, PET/CT image and clinical image of a tonsillar squamous cell carcinoma (arrows, patient no. 8). Right, overview of multiple generations of similar tumors from same patient. Insert is a picture of a mouse with a tumor established subcutaneously on the right hind leg for radiotherapy experiments.
Molecular characterization
All primary tumors, as well as PDX tumors from first and latest generations, were subjected to multiple analyses to characterize primary tumors, compare with PDX tumors, and assess the biological stability of PDX tumors over generations. These analyses are described in detail in the Supplementary methods. Briefly, tumors were first examined by conventional histology and immunohistochemistry, and p16 and HPV-status were determined. Next, expression of a number of genes related to hypoxia, radiosensitivity, gene expression subtype and cancer stem cell markers were examined [Citation11–16]. Last, we performed targeted tumor DNA sequencing of 409 cancer related genes, explained in detail in the Supplementary methods.
Radiosensitivity studies
Radiosensitivity was studied by a growth delay assay. For each PDX model, tumors were established subcutaneously in the right hind leg of 8 mice, which were assigned to receive 0, 4, 6 or 8 Gy as a single fraction, at a tumor volume of approximately 200 mm3. Mice were immobilized under a lead cover, exposing only the tumor-bearing leg to radiation. Radiation was delivered as 9 MeV electron irradiation from a clinical linear accelerator. A bolus of 15 mm was applied over the tumor to ensure uniform dose distribution.
Ethics
This study was approved by the Regional Health Ethics Committee and the Danish Animal Experiments Inspectorate.
Results
PDX establishment
From the 34 patients enrolled in the study, we established 12 squamous cell carcinoma PDX models, yielding a final take rate of 35% (). An additional 8 PDX models were found to be Epstein-Barr Virus (EBV) related lymphomas, and were discarded. In most cases of successful engraftment, multiple tumors were established and further xenotransplantation was uniformly successful.
Figure 2. Patient characteristics, and histological and immunohistochemical features of primary and PDX tumors over multiple generations. Green boxes in the last column indicates the 12 SCC PDX models established and further studied.
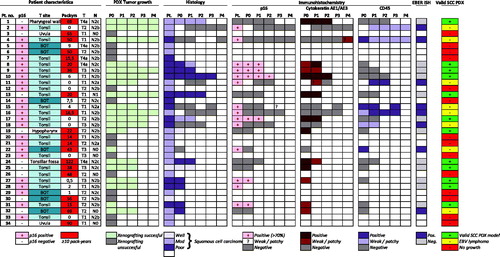
The established PDX models generally represented the clinical characteristics of the primary cohort well, although there were no base of tongue tumors or N0 tumors among the established PDX models (Supplementary Table 1).
Histology and immunohistochemistry
Overall, characteristics of established PDX models were found to be concordant with that of the primary tumor regarding histology, immunohistochemistry and HPV-status.
Established PDX models showed a tumor differentiation consistent with that of the primary tumor. This was also reflected in expression of p16 and the squamous differentiation marker cytokeratin AE1/AE3, as detected by immunohistochemistry ( and ).
Table 1. Overview of established SCC PDX models, patient characteristics, and selected molecular analyses.
Cancer gene targeted DNA sequencing
The PDX tumors and their original tumors showed high concordance with regards to specific gene variants. The genetic makeup of tumors was consistent with finding from other larger studies [Citation17,Citation18]. This included pathogenic TP53 mutations in all HPV-negative tumors, and KMT2D and PIK3CA mutations in HPV-positive tumors ().
Figure 3. Cancer gene variants in PDX models and original tumors. Only most frequently mutated genes are shown, with most important genes in bold (left). Columns represent individual tumors, primary and PDX of first and a later generation (P2-P4). Colors indicate type of mutation, see legend (right).
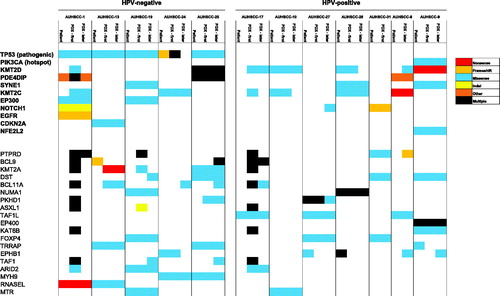
All mutations in important cancer driver genes in primary tumor samples were preserved in first passage PDX samples. Of these mutations, 88% of were preserved in later passage PDX tumors ( and Supplementary Figure 1(A)). Pathogenic TP53 mutations were found in all five HPV-negative primary tumors, and were found to be preserved in PDX models (). The number of mutations in first generation and later generation PDX tumors were slightly increased, compared to the human original tumors (p = .11 and 0.13. Supplementary Figure 1(B)).
Gene expression
We further characterized the biological stability of the PDX models by gene expression analyses (Supplementary Figures 2 and 3). We observed a moderate correlation between primary and PDX tumors with regards to expression of cancer stem cell markers MET and SLC3A2. PDX tumors had lower expression of PD-L1 and genes related to the previously described inflamed/mesenchymal gene expression subtype [Citation14]. PDX tumors had a notably higher hypoxia-related gene expression ( and Supplementary Figure 2).
Radiosensitivity
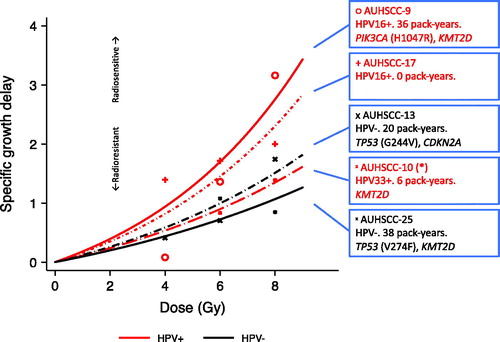
Growth delay assays were performed in 5 PDX models, 3 HPV-positive and 2 HPV-negative. Low-dose single-fraction irradiation of 4 – 8 Gy resulted in tumor regrowth delay in a dose-dependent fashion in all 5 PDX models (Supplementary Figure 4). Comparison of specific growth delay showed, that two HPV-positive PDX models were the most radiosensitive, as expected. However, one HPV-positive PDX-model was as radioresistant as the two HPV-negative. Interestingly, this patient has also developed an in-field tumor recurrence after curatively intended radiotherapy (68 Gy), indicating clinical radioresistance. The other patients had complete and durable responses ().
Figure 4. Results from a growth delay study performed in PDX tumors. Graphs show specific growth delay as a function of radiation dose (Shown in more detail in Supplementary Figure 5). A higher growth delay indicates greater radiosensitivity. All patients had lymph node-positive tonsillar squamous cell carcinoma. Red are HPV+, black are HPV−. Boxes provide HPV-status and -type, pack-years of tobacco smoking for each patient, as well as notable cancer driver gene mutations. (*) Patient AUHSCC-10 developed an in-field recurrence following radiotherapy.
Discussion
We have established a range of PDX models that reflect the spectrum of OPSCC with regards to HPV-status, smoking status and molecular characteristics.
PDX tumors have many similarities to the human original. Established PDX models maintain histological and immunohistochemical characteristics, HPV-status, important cancer driver gene mutations as well as expression of key genes. These findings are in line with findings from other groups [Citation19–21]. Further, preservation of specific cancer gene mutations detected by DNA sequencing serves to confirm the origin of the PDX tumor [Citation22].
We also found notable differences between primary and PDX tumors. PDX tumors acquire further genetic events during development, probably as a consequence of evolving tumor growth, similar to what is observed in human cancer [Citation23]. We also observed higher hypoxia-related gene expression, which could point to differences in tumor microenvironment, perhaps as consequence of implantation to the relatively avascular subcutaneous space. Finally, PDX tumors had lower expression of PD-L1 and genes related to the inflamed/mesenchymal subtype, which we ascribe to the fact that tumors were established in severely immunodeficient mice.
We observed that some tumors were not squamous cell carcinomas as expected, but instead EBV-positive lymphomas. This could be a consequence of a latent EBV-infection in the lymphoid tissue of Waldeyer’s throat ring, that might lead to lymphoma formation once these cells are transplanted to an immune-deficient host. This was also observed in another study of head and neck cancer PDX [Citation24], and may also be observed in humans as Post-Transplant Lymphoproliferative Disorder, which may occur with iatrogenic immunosuppression. Unfortunately, this phenomenon reduces the effective take-rate when generating PDX models, but the problem is probably restricted to tonsil and base of tongue tumors.
Our PDX models proved to be suitable for radiotherapy experiments, with consistent and dose-dependent growth delay. One should note, however, that growth delay studies probably reflect the most radiosensitive fraction of tumor cells, and as such is a rather rough measure. The more clinically relevant radioresistant fraction of tumor cells, as well as effects of hypoxia and fractionation, is not evaluated in this assay.
In our PDX models, experimental radiosensitivity was greater in HPV-positive tumors, which correlates well with the well-established better prognosis of these patients after curative radiotherapy [Citation3,Citation4]. There was, however, some variation in radiosensitivity of the HPV-positive tumors, and we noted a correlation with the clinical course of our patients. This suggests a correlation between experimental and clinical radiosensitivity, which highlights the clinical relevance of the PDX model. Furthermore, it supports a hypothesis of variable intrinsic radiosensitivity in HPV-positive OPSCC, which may be relevant in a clinical setting, and warrants further investigation in the context of the current de-escalation efforts in these patients.
Future perspectives includes expanding the number of PDX models, and adding tumor control studies, which may more accurately reflect clinical prognosis. Furthermore, the high fidelity of the PDX model could be increased by establishing PDX tumors in humanized mice with a functioning human immune system [Citation25]. This could reconstitute a tumor microenvironment with higher resemblance to the human primary tumor, and enable studies of interaction between tumor and immune system, as well as immunotherapy. The perspectives of such efforts would be increased insights into disease biology, and development of clinically relevant biomarkers.
In conclusion, we have established a range of patient-derived xenografts from head and neck squamous cell carcinomas, including HPV-positive oropharyngeal cancers. PDX tumors reflected the heterogeneity of patients regarding HPV and smoking status. PDX tumors preserved many characteristics in terms of histology, gene expression and important cancer driver gene mutations. There were some differences with regards to tumor microenvironment, specifically hypoxia and factors related to the immunosuppressed state of the mouse host. Our radiosensitivity studies showed concordance with the observed treatment response in clinical practice, underscoring the relevance of the PDX model in radiotherapy of head and neck cancer. On this basis, we find the PDX model to be an attractive pre-clinical surrogate cancer treatment model. Perspectives include increased understanding of disease biology, ultimately leading to the development of novel treatments and biomarkers.
Supplemental Material
Download PDF (496.1 KB)Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Lassen P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol. 2010;95(3):371–380.
- Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–3242.
- Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(12):1992–1998.
- Lassen P, Lacas B, Pignon J, et al. Prognostic impact of HPV-associated p16-expression and smoking status on outcomes following radiotherapy for oropharyngeal cancer: the MARCH-HPV project. Radiother Oncol. 2018;126(1):107–115.
- Bentzen J, Toustrup K, Eriksen JG, et al. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncol. 2015;54(7):1001–1007.
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
- Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–350.
- Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013;73(17):5315–5319.
- Willey CD, Gilbert A, Anderson JC, et al. Patient-derived xenografts as a model system for radiation research. Semin Radiat Oncol. 2015;25(4):273–280.
- Byrne AT, Alférez DG, Amant F, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17(4):254–268.
- Sørensen BS, Toustrup K, Horsman MR, et al. Identifying pH independent hypoxia induced genes in human squamous cell carcinomas in vitro. Acta Oncol. 2010;49(7):895–905.
- Toustrup K, Sørensen BS, Nordsmark M, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71(17):5923–5931.
- Toustrup K, Sørensen BS, Metwally MAH, et al. Validation of a 15-gene hypoxia classifier in head and neck cancer for prospective use in clinical trials. Acta Oncol. 2016;55(9–10):1091–1098.
- Keck MK, Zuo Z, Khattri A, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870–882.
- Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75(2):489–496.
- Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–211.
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582.
- Gillison ML, Akagi K, Xiao W, et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. 2019;29(1):1–17.
- Kimple RJ, Harari PM, Torres AD, et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumorgrafts. Clin Cancer Res. 2013;19(4):855–864.
- Keysar SB, Astling DP, Anderson RT, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 2013;7(4):776–790.
- Joshua B, Kaplan MJ, Doweck I, et al. Frequency of cells expressing CD44, a head and neck cancer stem cell marker: correlation with tumor aggressiveness. Head Neck. 2012;34(1):42–49.
- Lilja-Fischer JK, Saksø M, Stougaard M, et al. Distinguishing recurrence and new primary tumor as well as the origin of neck metastases in head and neck cancer clinical trials by targeted DNA sequencing. Acta Oncol. 2019;58:1506–1508.
- Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446–451.
- Facompre ND, Sahu V, Montone KT, et al. Barriers to generating PDX models of HPV-related head and neck cancer. Laryngoscope. 2017;127(12):2777–2783.
- Morton JJ, Keysar SB, Perrenoud L, et al. Dual use of hematopoietic and mesenchymal stem cells enhances engraftment and immune cell trafficking in an allogeneic humanized mouse model of head and neck cancer. Mol Carcinog. 2018;57(11):1651–1663.
