Abstract
Background: There have been large changes in the pattern of detection, work-up and treatment of men with prostate cancer during the last two decades. Therefore, we aimed to investigate temporal changes in survival in men with metastatic prostate cancer.
Methods: Population-based cohort study in Prostate Cancer data Base Sweden of 13,709 men with de novo metastatic prostate cancer diagnosed between 1998 and 2015. Overall survival in four calendar periods were compared by the use of Kaplan–Meier analyses and Cox regression models including age at diagnosis, T stage and serum levels of prostate-specific antigen (PSA).
Results: Between 1998–2001 and 2010–2015, median survival increased with 6 months for all men. The largest increase in survival was 14 months in men age 60–69 at diagnosis and in multivariable analysis risk of death decreased for men diagnosed in 2010–2015 compared to 1998–2001, hazard ratio (HR) 0.77 (95% CI: 0.68–0.86). The median PSA at date of diagnosis decreased with 46% from 181 ng/mL in 1998 to 98 ng/mL in 2015.
Conclusions: There was an increase in survival among men with de novo metastatic prostate cancer in Sweden between 1998 and 2015. This increase was due to a decreased cancer extent indicated by lower PSA levels with ensuing longer lead times and speculatively also due to an increased use of chemotherapy in the latest time period. Given the increasing use of systemic treatment for advanced prostate cancer, our results are likely heralding larger increases in survival in men with metastatic prostate cancer in the near future.
Introduction
There have been major changes in prostate cancer detection, work-up and treatment during the last two decades [Citation1,Citation2], with a strong increase in use of prostate-specific antigen (PSA) testing for prostate cancer detection [Citation3] with an increased detection of low-risk cancer resulting in longer lead time from diagnosis to death [Citation4,Citation5]. There have also been changes in the Gleason classification leading to a grade migration [Citation6].
Androgen deprivation therapy (ADT) remains the cornerstone of treatment of men with metastatic prostate cancer. In addition, chemotherapy and several other new classes of drugs have been shown to prolong survival for men with castration-resistant prostate cancer and more recently also hormone sensitive metastatic prostate cancer and these results have changed guidelines [Citation7–10]. In a study of men who died of prostate cancer in 2009–2010 in Sweden [Citation11], as well as in a more recent audit of men diagnosed in 2010–2016 (data on file), slightly less than half of men below age 80 received chemotherapy whereas only 5% of men above age 80 received chemotherapy.
Against this backdrop of strong changes in detection, diagnostics and treatment of prostate cancer, we aimed to investigate survival in men with de novo metastatic prostate cancer, i.e., metastases detected in the initial work-up, in a nationwide population-based cohort.
Material and methods
Data on men with prostate cancer diagnosed between 1998 and 2015 were retrieved from the National Prostate Cancer Register (NPCR) of Sweden [Citation12], a nationwide clinical cancer register that captures data on cancer characteristics according to the TNM classification, Gleason grading reported with the five-tiered Gleason Grade Groups (GGG) [Citation13], serum level of PSA, diagnostic work-up and primary treatment. Data linkages in the Prostate Cancer data Base Sweden (PCBaSe) have been performed between NPCR and other healthcare registers and demographic databases [Citation14]. By use of the Swedish person identity number, data from NPCR were cross-linked to data from the Patient Registry, the Prescribed Drug Registry, the Swedish Cancer Registry, the Cause of Death Registry, and the Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA). Comorbidity at date of diagnosis was defined by use of the Charlson Comorbidity Index (CCI), which is a weighted sum of a number of diagnostic codes for discharge diagnoses, including other prior cancers, but excluding prostate cancer [Citation15]. Information on other cancer diagnoses was retrieved from the Cancer Registry, date and cause of death were retrieved from the Cause of Death Registry, and educational level (low = less than 10 years, intermediate = 10–12 years, high = more than 12 years of education) and marital status were retrieved from the LISA database, a socioeconomic database held at Statistics Sweden. Mode of detection of the Pca was categorized as symptomatic (lower urinary tract symptoms or other symptoms) or asymptomatic. Follow-up was obtained until death, censoring or 2016-12-31.
In NPCR, M stage indicates presence of metastases on imaging. Current Swedish guidelines recommend against bone imaging in men with low and intermediate-risk prostate cancer [Citation16]. Therefore, metastatic status according to bone imaging, i.e., non-metastatic or metastatic prostate cancer, was not available for all men and was considered to be ‘missing not at random’ (MNAR).
Overall survival of men diagnosed with metastatic prostate cancer in four calendar periods (1998–2001, 2002–2005, 2006–2009 and 2010–2015) and according to age at diagnosis was investigated by use of Kaplan–Meier curves, and other cause mortality was investigated using competing risk analysis and cumulative incidence curves. Three Cox Proportional Hazards regression models for all-cause mortality were used. The univariable model included calendar period of diagnosis only, the first multivariable model controlled for symptomatic or asymptomatic status at start of work-up leading to prostate cancer diagnosis, clinical T stage, serum PSA, age at diagnosis, CCI and educational level, and the second additionally controlled for Gleason grading [Citation17].
Since the proportion of men with missing data on M stage was high (66%) we used Multiple Imputation through Chained Equations (MICE) to reconstruct missing data [Citation18]. The imputation model included all above-mentioned variables, and the number of multiple imputations was set to 20 with 10 iterations. We aimed to impute the clinical stages ‘metastatic prostate cancer’ and ‘non-metastatic prostate cancer’, corresponding to the results of a bone imaging under the hypothetical scenario in which all men had undergone such an investigation. A binary nonignorable marginal imputation model was used to account for informative missingness [Citation19]. In short, the strength of the association is expressed through a constant multiplier k which describes the odds of metastatic prostate cancer for men with nonignorable missing data compared to men with ignorable missing data. Men with lower Gleason grades were assumed to have a higher degree of informative missingness in favor of non-metastatic prostate cancer so the multiplier k was allowed to depend on Gleason Grade Groups, with k = 0.1 for GGG 1, GGG 2: k = 0.3, GGG 3: k = 0.6 and GGG 4–5: k = 0.7 [Citation20]. A sensitivity analysis using different values for k was also performed. All analyses were compared with a complete case analysis [Citation18]. The analysis was performed using R 3.4.2. The study was approved by the Research Ethics Board in Uppsala.
Results
Baseline characteristics of men with metastatic prostate cancer
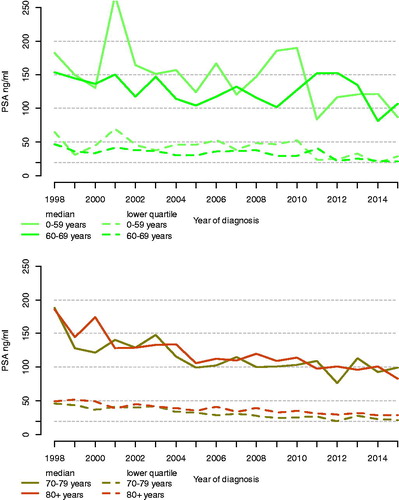
There were 13,709 (9%) men with positive bone imaging (M1) and 105,907 (66%) men who had not undergone bone imaging (Mx). After imputation, there were 21,734 (14%) men with metastatic prostate cancer. Most cancer characteristics of men with known metastatic prostate cancer were fairly constant over calendar years, apart from Gleason and PSA ().
Table 1. Baseline characteristics for men with de novo metastatic prostate cancer in Prostate cancer data Base Sweden (PCBaSe) 4.0.
Temporal changes in PSA levels and Gleason grading
The median PSA at date of diagnosis decreased from 181 in 1998 to 98 ng/mL in 2015 (46%), for men below age 60, from 182 in to 87 ng/mL (52%), for men age 60–69, from 153 to 107 ng/mL (30%), for men age 70–79 from 188 to 99 ng/mL (47%), for men age 80–90 from 185 to 83 ng/mL (55%) and for men age 90+ from 172 ng/mL to 167 ng/mL (3%) (, Supplementary Figure 1). There was a higher proportion of high Gleason grades in more recent calendar periods compared to earlier periods and this increase remained also after standardization (Supplementary Figure 2). For example, the number of men with GGG 1 decreased from 272 (20%) in 1998 to 36 (3%) in 2015, and the number of men with GGG 5 increased from 424 (31%) to 572 (49%) (Supplementary Table 1).
Survival
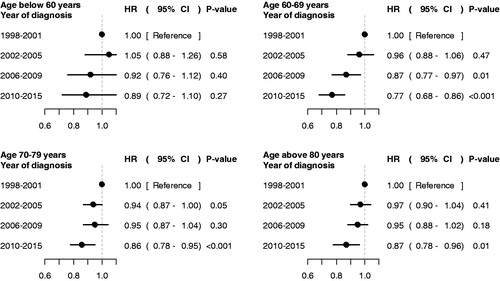
There was an increase in median overall survival between 1998–2001 and 2010–2015 of 6 months, from 2.2 years (95% CI: 2.1–2.4) to 2.7 years (95% CI: 2.6–2.9). The largest increase was observed in men 60–69 years old at diagnosis for whom survival increased with 14 months from 2.6 years (95% CI: 2.4–2.9) to 3.8 years (95% CI: 3.5–4.2) and the increase was most pronounced between the two last calendar periods (; Supplementary Figure 3). A smaller increase in survival of 6 months was observed among men less than age 60 at diagnosis, up from 2.5 years (95% CI: 2.2–2.8) to 3.0 years (95% CI: 2.7–3.5), for men 70–79 the increase was 7 months up from 2.4 years (95% CI: 2.2–2.5) to 3 years (95% CI:2.8–3.2), for men 80–89 the increase was 4 months from 1.8 years (95% CI: 1.6–2.0) to 2.2 years (95% CI: 2.0–2.3) and for men above 90 the increase was 4 months, up from 1.2 years (95% CI: 0.9–1.6) to 1.5 years (95% CI: 1.3–1.7). Similar increases were also observed in 3-year survival (Supplementary Figure 4; Supplementary Table 2). Corresponding hazard ratios (HR) for death from all causes in multivariable analysis for men diagnosed in 2010–2015 compared with 1998–2001 showed that these increases remained after adjustments, risk for men below age 60, HR 0.89 (95% CI: 0.72–1.1), age 60–69, HR 0.77 (95% CI 0.68–0.86), age 70–79, HR 0.86 (95% CI 0.78–0.95) and age above 80, HR 0.87 (95% CI: 0.78–0.96) (; Supplementary Figure 5, Table 3). These results remained essentially unchanged in the complete case analysis (Supplementary Figures 1–5) and in the sensitivity analysis of various levels of the multiplier k (data not shown). Finally, death from other causes than prostate cancer was stable across calendar periods in most age groups, apart from a slight reduction for younger men so almost all of the increase in survival was due to a reduced prostate cancer-specific mortality (Supplementary Figure 6).
Figure 2. Median overall survival by calendar period in men with metastatic prostate cancer in Prostate Cancer data Base Sweden (PCBaSe) 4.0. Vertical bars indicate 95% confidence intervals.
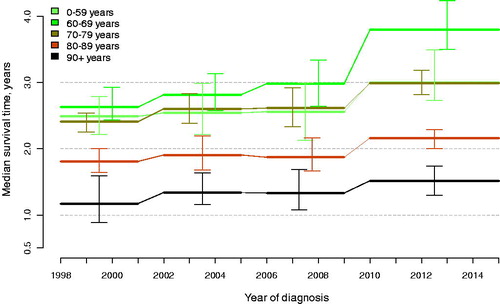
Figure 3. Overall survival for men with metastatic prostate cancer in PCBaSe 4.0. Hazard ratios (HR) in Cox proportional hazard regression model for overall survival in men with metastatic prostate cancer according to calendar period, controlling for symptomatic or asymptomatic status at start of work-up leading to prostate cancer diagnosis, serum level of prostate specific antigen (PSA), clinical T stage, age at diagnosis, Charlson comorbidity index and educational level.
Discussion
In this nationwide, population-based study in Sweden of men with de novo metastatic prostate cancer diagnosed between 1998 and 2015, we observed a 6-month increased median survival. This increase was largest in men aged 60–69, for whom survival increased 14 months, whereas men below 60 years and men aged 70–79 had smaller increases of 6–7 months, and men above 80 had an even smaller increase. Strong decreases in median PSA during the study period indicating smaller cancer extent were observed in all age groups except in men above 90 so longer lead times was a main contributor to the increase in survival. However, the introduction of chemotherapy during the latest time period may also have contributed to the increase [Citation11].
Limitations of our study include lack data on use of chemotherapy since this is not recorded in The Prescribed Drug Registry in which only drugs that are delivered by filling of prescriptions are recorded. In a previous survey of men who died of prostate cancer in 2009–2010, 485 out of 1191 (41%) below age 80 had received chemotherapy, and in a more recent survey of 694 men below 80 diagnosed with metastatic prostate cancer in 2010–2016 48% had received chemotherapy (data on file). Based on these numbers, we estimate that in the most recent calendar period, slightly less than half of men below 80 years likely received chemotherapy sometime during their disease trajectory. In both of these studies, only around 5% of men above age 80 received chemotherapy, so likely very few men in this age bracket received chemotherapy in our study. Moreover, data on filled prescriptions for abiraterone and enzalutamide were only available in the Swedish Prescribed Drug Registry as from July 2015, and therefore not included in the present study.
We observed strong changes in the Gleason grading during the study period with an increased use of higher grades which was likely due to a grade inflation, in part caused by a change in the ISUP classification, and by a higher number of biopsy cores obtained at transrectal ultrasound examination [Citation13]. This change most likely caused us to overestimate the effect of calendar period on survival when we controlled for Gleason. Thus, the improvement in survival is best assessed without controlling for Gleason grade. Another limitation of our study is the relative short follow-up time of 1–3 years for men diagnosed in 2014–2015 in comparison to the median survival of 1.5–3.8 years observed in 2010–2015.
In a previous study in PCBaSe, men with missing data had a lower prostate cancer risk category than men with complete data [Citation20]. In support of this view, higher PSA levels were observed in men who had undergone bone imaging than in men for whom this investigation was omitted. Thus, data on metastatic status were missing not at random and we addressed this issue by use of a binary nonignorable marginal imputation model to account for informative missingness [Citation19]. Results from the imputed data, sensitivity analysis and complete cases were similar, suggesting that missing data only had a small effect on the results and no effect on our conclusion.
Our study was based on data from a very large number of men of whom a proportion had undergone imaging, most of them a bone scan. We cannot exclude that the sensitivity of the test has increased due to improvement imaging technique and interpretation of the images, however the relatively stable proportion of men diagnosed with metastatic prostate cancer verified by imaging indicates that no major changes occurred during the study period.
A major strength of our study was that we had access to data from a comprehensive, nationwide population-based, high-quality clinical cancer register including almost complete data on PSA levels and clinical T stage at date of diagnosis. PSA levels decreased substantially over time. Since the methods for PSA measurements have remained essentially unchanged during the study period, this is a strong indicator of a decreasing tumor burden in men with de novo metastatic prostate cancer. We also had access to data from other healthcare registers and demographic databases with documented high quality, e.g., high accuracy for discharge diagnoses in the Patient Registry for virtually all men with prostate cancer in Sweden [Citation14, Citation21–23]. In addition, we used death from all causes as the primary end-point thereby avoiding problems with assessment of cause of death. However, the increased survival was mainly due to reduced prostate cancer-specific mortality since mortality from other causes remained stable in most age groups during the study period.
A previous study reported an increase in survival in men with metastatic prostate cancer in Denmark whereas in The Surveillance, Epidemiology and End Results (SEER) cohorts in the US no change in survival had occurred between 1980 and 2008 [Citation24]. A more recent update of the SEER cohort showed a slightly increased survival in men with de novo metastatic prostate cancer [Citation25]. These studies did not include data on PSA and Gleason grade so cancer extent and cancer differentiation could not be assessed, nor was there any indication on use of chemotherapy.
Compared to men in the control arm in recent randomized clinical trials on chemotherapy combined with ADT for hormone-naïve prostate cancer, men in our study were older, median age at diagnosis 76 years, versus median age at inclusion to these trials which was 63–66 years [Citation7, Citation26–28]. Survival was longer in the control arm of these RCT’s, 42–71 months after inclusion than in our study. Men in these trials also had lower median PSA levels (26–112 ng/mL), indicating a smaller tumor burden compared to our study group. These differences illustrate that men included in a RCT are a selection of younger and overall healthier men with less cancer extent compared to men in routine clinical practice, aka ‘real world’.
Given that likely less than half of all men with de novo metastatic prostate cancer in our study received chemotherapy sometime during their disease trajectory even in the most recent calendar period [Citation11], continued improvements in survival are to be expected. In randomized clinical trials, increased survival has been shown in men treated with chemotherapy for CRPC as well as upfront for hormone-naïve metastatic prostate cancer [Citation27,Citation28] and in men treated with other novel drugs including androgen receptor targeted drugs both in the castration-resistant state and in earlier phases of the disease [Citation8,Citation9].
Conclusion
In this nationwide, population-based study in Sweden, overall survival in men with de novo metastatic prostate cancer increased between 1998 and 2015. Contributing factors to this increase were smaller tumor burden as indicated by decreasing PSA levels with calendar time, with ensuing longer lead times, and use of chemotherapy could also have contributed to increased survival in the latest time period. It is important to assess grade inflation and cancer extent when comparing survival of men with metastatic prostate cancer across time periods. Finally, there is good hope that this is just the beginning of an increase in survival in men with metastatic prostate cancer since several other life-prolonging treatments for metastatic prostate cancer and castration-resistant prostate cancer have been approved and other novel drugs are currently tested in trials.
Supplemental Material
Download (292.4 KB)Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chair), Ingela Franck Lissbrant (co-chair), Camilla Thellenberg Karlsson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm-Eriksson, David Robinson, Mats Andén, Jonas Hugosson, Maria Nyberg, Ola Bratt, Calle Waller, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin, Karin Hellström.
Additional information
Funding
References
- Annual report. 2018. from The National Prostate Cancer Register. In Swedish. [cited 2019 Sep 09]. Available from: http://npcr.se/wp-content/uploads/2019/09/20190905_npcr_nationell_rapport_2018.pdf.
- Stattin P, Sandin F, Loeb S, et al. Public online reporting from a nationwide population‐based clinical prostate cancer register. BJU Int. 2018;122(1):8–10.
- Jonsson H, Holmstrom B, Duffy SW, et al. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2011;129(8):1881–1888.
- Ohmann EL, Loeb S, Robinson D, et al. Nationwide, population-based study of prostate cancer stage migration between and within clinical risk categories. Scand J Urol. 2014;48(5):426–435.
- Bray F, Lortet-Tieulent J, Ferlay J, et al. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46(17):3040–3052.
- Cazzaniga W, Garmo H, Robinson D, et al. Mortality after radical prostatectomy in a matched contemporary cohort in Sweden compared to the Scandinavian Prostate Cancer Group 4 (SPCG‐4) study. BJU Int. 2018;123: 421–428.
- Gravis G, Boher JM, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70(2):256–262.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433.
- Ryan CJ, Smith MR, De Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148.
- National guidelines for prostate cancer. In Swedish. [citedAug 20 2019]. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/prostatacancer/vardprogram/nationellt-vardprogram-prostatacancer.pdf.
- Lissbrant IF, Garmo H, Widmark A, et al. Population-based study on use of chemotherapy in men with castration resistant prostate cancer. Acta Oncol. 2013;52(8):1593–1601.
- RATTEN - Interactive On Line Report from NPCR [cited 2019 Aug 20]. Available from: https://statistik.incanet.se/npcr/.
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252.
- Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort Profile: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42(4):956.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Makarov DV, Loeb S, Ulmert D, et al. Prostate cancer imaging trends after a nationwide effort to discourage inappropriate prostate cancer imaging. J Natl Cancer Inst. 2013;105(17):1306–1313.
- Cox DR. Regression models and life-tables. J Roy Stat Soc B (Methodological). 1972;34(2):187–220.
- van Buuren S. Flexible imputation of missing data. Boca Raton (FL): CRC Press; 2012.
- Siddique J, Harel O, Crespi CM, et al. Binary variable multiple-model multiple imputation to address missing data mechanism uncertainty: application to a smoking cessation trial. Stat Med. 2014;33(17):3013–3028.
- Tomic K, Westerberg M, Robinson D, et al. Proportion and characteristics of men with unknown risk category in the National Prostate Cancer Register of Sweden. Acta Oncol. 2016;55(12):1461–1466.
- Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol. 2015;54(2):158.
- Tomic K, Sandin F, Wigertz A, et al. Evaluation of data quality in the National Prostate Cancer Register of Sweden (Reprinted). Eur J Cancer. 2015;51(9):1130–1130.
- Ingelsson E, Ärnlöv J, Sundström J, et al. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7(5):787–791.
- Helgstrand JT, Røder MA, Klemann N, et al. Trends in incidence and 5‐year mortality in men with newly diagnosed, metastatic prostate cancer—a population‐based analysis of 2 national cohorts. Cancer. 2018;124(14):2931.
- Bandini M, Pompe RS, Marchioni M, et al. Improved cancer-specific free survival and overall free survival in contemporary metastatic prostate cancer patients: a population-based study. Int Urol Nephrol. 2018;50(1):71–78.
- James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “Docetaxel Era”': data from 917 patients in the control arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur Urol. 2015;67(6):1028–1038.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746.