Abstract
Background: Metastatic disease in colorectal cancer represents a major cause of significant cancer-associated morbidity and mortality. L1CAM is a stem cell marker, cell adhesion molecule, belongs to the immunoglobulin superfamily of cell adhesion molecules (IgCAM) and it is aberrantly expressed in several different types of human solid tumors. The aim of the present study was to assess the expression patterns of L1CAM and its clinical significance in colorectal cancer.
Patients and methods: Surgical specimens of 109 patients with primary resectable colorectal cancer were examined for L1CAM expression via immunohistochemistry and the results were correlated with clinical and survival data.
Results: L1CAM expression was significantly correlated with advanced stage of disease (p < .001), higher T classification (p = .040), the presence of lymph node (p < .001) and distant metastasis (p = .011). Patients displaying high L1CAM expression demonstrated a dismal three-year progression free survival (29.7% vs 87.1%, p < .001) and five-year overall survival (39.9% vs 87.7%, p < .001). Multivariate analysis using Cox proportional hazard models revealed high L1CAM expression as a prognostic marker of dismal progression free (HR 0.187, 95%CI = 0.075–0.467, p < .0001) and overall survival (HR 0.154, 95%CI = 0.049–0.483, p = .001) independent of other clinicopathological characteristics. Subgroup analysis comprised of patients with early stage disease only presented as well significantly worse progression free and overall survival when L1CAM exhibited high expression.
Conclusions: Colorectal cancer patients displaying high expression of L1CAM harbor high risk for metastasis already in early stage disease identifying therefore a group of patients prone to dismal prognosis.
Introduction
Colorectal cancer (CRC) represents the third most common cancer and constitutes the second leading cause of cancer-associated death among adults in the western societies [Citation1]. Despite the improvement of multimodality treatment with surgical resection and chemo-radiation, when indicated [Citation2], there is still a significant cancer-associated morbidity and mortality that is mainly attributed to metastatic disease [Citation3]. A relatively large proportion of patients with nonmetastatic disease at the time of diagnosis, ranging between 14%–34% [Citation4], will develop metachronous metastases with 86% of them within the first three years [Citation5].
Basic steps of metastasis include invasion and intravasation of cancer cells from the primary tumor, dissemination through the circulation and extravasation in distant organs [Citation6]. Those that survive need to settle from latency, being reactivated and consequently generate a macroscopic tumor [Citation7–8]. These cells harbor properties of tumor-initiating cells/cancer stem cells and manage to settle under harshly adverse conditions. Therefore, cancer stem cells drive metastasis [Citation6].
L1CAM is a stem cell marker, cell adhesion molecule and belongs to the immunoglobulin superfamily of cell adhesion molecules (IgCAM). A recent review [Citation9] regarding L1CAM expression showed that it is aberrantly expressed in several solid tumors including CRC. The aim of this study was to assess the expression patterns of L1CAM in CRC and its clinical significance focusing on early-stage disease.
Material and methods
Patients and tissues specimens
A total of 109 clinically annotated surgically removed specimens from patients with CRC were identified during 2008 to 2010 from the files of the 2nd Department of Propedeutic Surgery of the Laiko General Hospital, National and Kapodistrian University of Athens, Greece. Written informed patient consent was obtained in advance. The Regional Ethics Committee (ethics committee of the National and Kapodistian University, Athens Medical School, Laiko General Hospital) approved the use of the tissue samples for translational research purposes and the study was conducted according to the Declaration of Helsinki and results are presented in accordance with the REMARK guidelines [Citation10].
Patients that underwent a curative surgical resection were included in the study. Therefore, a priori, patients with evidence of metastatic disease were included in the study population only when liver metastases occurred and a curative surgical resection was feasible in terms of a Wedge/anatomic resection.
Clinicopathological features
Clinicopathological data were identified from a prospectively collected database and were retrospectively analyzed in a nonstratified and nonmatched manner. Annotation included patient age, gender, location of the tumor, pT/pN classification, tumor grade, histologic subtype, vascular invasion, perineural invasion, the presence of metastasis and the clinical stage of the disease according to UICC classification.
Immunohistochemistry
Standard procedures were used for the preparation of the formalin-fixed, paraffin-embedded tissue blocks. Staining was done on regular 4 μm sections of formalin-fixed and paraffin-embedded tumor tissue. Immunohistochemistry was performed on tissue sections with the standard biotin–avidin complex technique using a rabbit monoclonal antibody (dilution 1:250) against L1CAM that was purchased from the Abcam biotechnology, Cambridge, United Kingdom.
Histopathological evaluation was performed by two experienced pathologists with expertise in the field (A.N.) and (E.P.). Both researchers were blinded to the clinical data of the patients. The intensity of staining was scored as follows: 0: no staining of the cells, 1: mild staining, 2: intermediate degree of staining, 3: strong staining intensity. Negative control was prepared by substituting the primary antibody over PBS. Only specimens with strong intensity were considered as positive for the expression of L1CAM. Using ROC curve cut off points were investigated and as a consequence, a specimen exhibiting L1CAM staining in less than 5% of the cancer cells was defined as a specimen with low or no expression of L1CAM. In the present study, a specimen was considered as positive for L1CAM expression when L1CAM staining displayed strong intensity and high expression (more than 5% of the cancer cells) simultaneously.
Statistical analysis
SPSS IBM statistics software (version 21.0) was used to perform the statistical analysis of the current study. X2 test was deployed to investigate correlations among L1CAM expression and the clinicopathological characteristics. The application of 2-sided chi-square and Fisher’s exact test assessed whether significance existed. The time of occurrence of disease recurrence was defined as the time in months after surgery until the occurrence of the first relapse manifesting either locally or with distant disease. The cumulative survival probabilities were estimated by using the Kaplan–Meier curves. Finally, data related to the L1CAM expression were entered into multivariate Cox regression analysis, while hazard ratios (HR) and 95% confidence intervals (CI) were used to determine prognostic effects on survival time. A p-value of < 0.05 was considered statistically significant.
Primary outcome
Progression-free survival (PFS) and overall survival (OS) were defined as the primary outcomes of this study.
Results
L1CAM expression and association with clinicopathological characteristics
L1CAM displayed a strong expression in the cytoplasm of the colorectal cancer cells (). of the supplementary material summarizes the descriptive characteristics of the study population. Statistically significant correlations were demonstrated among positive L1CAM expression and advanced stage of disease (III + IV, p < .001), tumors penetrating the surface of the visceral peritoneum (T4, p = .040), the presence of lymph node metastasis (p < .001) and metastatic disease (p = .011). On the contrary, no significant associations were noted among high L1CAM expression and location of the tumor, tumor grade (p = .647), vascular invasion (p = .360), perineural invasion (p = .272) or the presence of mucinous histologic subtype (p = .717). A summary of the reported correlations is depicted in .
Figure 1. Intense cytoplasmic staining of neoplastic and membranous cells for L1CAM (A) (X100). (B) (X200). (C) Absence of L1CAM staining in normal colonic epithelium. There is L1CAM staining in the ganglionic cells (x200).
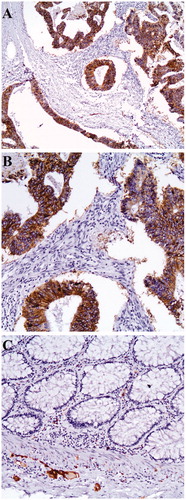
Table 1. Correlation of L1CAM expression with clinicopathological characteristics.
Survival analysis and prognostic significance of L1CAM expression
Patients exhibiting high expression of L1CAM demonstrated statistically significant higher rates of disease recurrence or death demonstrating a 3-year PFS () that reached only 29.7% (95% CI = 21–49) versus 87.1% (95% CI = 77–93) for patients with low expression of L1CAM (p < .001). Regarding OS, patients with a high expression of L1CAM presented a significantly worse 5-year OS () reaching only 39.9% (95% CI = 36–65), versus 87.7% (95% CI = 84–97) for patients with low expression of L1CAM (p < .001). Multivariate analysis using Cox proportion hazard models identified high expression of L1CAM as a prognostic factor of PFS (HR 0.187, 95% CI = 0.075–0.467, p < .0001, ) and OS (HR 0.154, 95% CI = 0.049–0.483, p = .001, ) independent of TNM stage, grade of cellular differentiation of the cancer cells, vascular and perineural invasion. Consequently, the risk of occurrence of disease recurrence or death in the first 3 years after operation increases by almost 5.4-fold while the risk of death in the first 5 years after operation increases by almost 6.5 when high L1CAM expression exists.
Figure 2. Kaplan–Meier curve depicting progression-free survival (A) and overall survival (B) in months of the study population according to the levels of expression of L1CAM (low vs high). Kaplan–Meier curve illustrating progression-free survival (C) and overall survival (D) of patients with early stage cancer only (stage I and II) and low versus high expression of L1CAM.
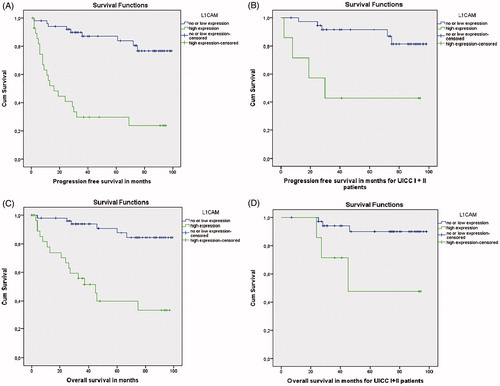
Table 2. Multivariate analysis regarding progression free survival (PFS) and overall survival (OS) using cox proportional hazard models.
Subgroup analysis comprised of patients with early-stage disease (UICC I + II) only confirmed a statistically significant worse 3-year PFS reaching 42.9% (95% CI = 19–78) in patients exhibiting high expression of L1CAM versus 91.2%, (95% CI = 81–96) for patients with low L1CAM expression (p = .003). In addition 5-year OS was statistically significantly poor for patients with high expression of L1CAM (47.6%, 95% CI = 37–65, versus 90%, 95% CI = 85–98 respectively, p = .008).
Discussion
In the present study, high expression of L1CAM was associated with aggressive clinical features and poor three-year PFS and five-year OS in the study population and in patients with early-stage cancer only regardless of clinicopathological results.
Similar results regarding the aggressive nature of cancer cells expressing L1CAM have been supported previously in the literature based on a series of studies on solid tumors. More specifically, aggressive clinical features have been previously reported for the following malignancies; ovarian, prostate, renal cell carcinoma, nonsmall cell lung cancer, mesothelioma, pelvic tumor of unknown origin, breast and triple negative cancers, melanoma, anaplastic thyroid carcinoma, glioblastoma, neuroblastoma, cholangiocarcinoma, esophageal cancer, pancreatic ductal adenocarcinoma and neuroendocrine tumors, gallbladder, hepatocellular carcinoma, GIST, gastric and colorectal [Citation9].
In the present study, L1CAM was correlated with metastasis and poor overall survival already in early-stage disease. An experimental study [Citation11] on mouse models demonstrated that L1CAM in human colon cancer cells enables metastatic capacity to the liver after injecting tumor cells into the spleen of the mice, via a mechanism mediated from the metalloproteinase ADAM10 that cleaves the L1CAM extracellular domain, as target gene of β-catenin-T-cell-factor signaling [Citation12]. Another study [Citation13] on transfected mice showed that changes in epithelial to mesenchymal transition (EMT) is not a requirement of L1CAM-mediated metastasis of colorectal cells and that L1CAM induced metastatic pathway by activating Nuclear factor-κβ signaling [Citation14].
It has been recently demonstrated [Citation15] that perivascular spreading and outgrowth of metastasis-initiating cells is promoted by an L1CAM-mediated mechanism similar to the one that pericytes use in order to maintain vascular homeostasis in cases of vascular stress and neoangiogenesis. More specifically, metastatic colonization implicates an organ-specific interplay between disseminated cancer cells and their surrounding tumor stroma, promoting a diversity of interactions [Citation16]. Disseminated cancer cells are persistently localized at the perivascular niche which provides them access to oxygen, nutrients and endothelium-derived paracrine factors that further promote self-renewal, proliferation and survival [Citation17–18], affecting metastatic activity [Citation19–20]. The latter, nevertheless, is not sufficient to promote metastatic spread. Interestingly, in vivo experiments have demonstrated that L1CAM-deficient cells after extravasating in the brain parenchyma can still invade the perivascular niche but their growth and spread cannot occur [Citation21]. An L1CAM mediated activation of the mechanotransduction effectors YAP (Yes-associated protein) and MRTF (myocardin-related transcription factor) is required to enhance disseminated cancer cells to spread over the abluminal surface of blood vessels and therefore displace resident pericytes, which also use L1CAM for perivascular spreading [Citation15]. These signaling pathways provoke the outgrowth of metastasis- initiating cells either directly by allowing them to infiltrate their target organs or after they exit them from a period of latency [Citation15].
The clinical significance of the presented data raises questions whether a high-risk group of patients prone to metastasis in colorectal cancer could be identified already in early-stage disease and potentially treated according to a targeted therapy. In the present study, three-year PFS of patients with early-stage disease was only 42.9%. It has been supported that approximately 10% of the patients with stage I and 20% of the patients with stage II will present disease recurrence [Citation22]. The use of adjuvant chemotherapy in patients with stage-II colonic cancer remains controversial due to minimal survival benefit and often high toxicity of the drugs [Citation23]. Furthermore, previous studies that aimed to identify the subgroup of patients with stage II cancer that are at increased risk of recurrence are not vigorous enough [Citation24] to warrant a clear indication for adjuvant treatment. An issue to be addressed is whether patients with high L1CAM expression benefit from conventional chemotherapy or whether a targeted therapy in terms of a monoclonal antibody should be developed. Targeted therapy against L1CAM for cancer has been evaluated before with antibodies [Citation25–27]. In ovarian cancer, they inhibited growth and dissemination of ovarian carcinoma cells in the peritoneal cavity. Treatment of pancreatic and ovarian xenografts using the combination of L1CAM antibodies and cytotoxic drugs displayed increased therapeutic response [Citation28].
Combined all together patients demonstrating high expression of L1CAM appear to harbor a significant risk for cancer associated mortality already from an early-stage disease. Randomized clinical trials assessing the efficacy of a targeted therapy against L1CAM in colorectal cancer need to be recruited. The use of adjuvant therapy either as conventional chemotherapy or targeted therapy in early stage cancer remains a question to be answered.
There are limitations in the present study which mainly originate from the retrospective nature of the study, the sample of the study population and the heterogeneity of L1CAM expression within the tumor that might be underestimated with the use of immunohistochemistry.
Supplemental Material
Download MS Word (31.6 KB)Disclosure statement
The authors declare no conflict of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502.
- Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18(1–2):43–73.
- Guyot F, Faivre J, Manfredi S, et al. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol. 2005;16(5):756–761.
- van Gestel YR, de Hingh IH, van Herk-Sukel MP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. 2014;38(4):448–454.
- Oskarsson T, Batlle E, Massague J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14(3):306–321.
- Janni W, Vogl FD, Wiedswang G, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse–a European pooled analysis. Clin Cancer Res. 2011;17(9):2967–2976.
- Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802.
- Altevogt P, Doberstein K, Fogel M. L1CAM in human cancer. Int J Cancer. 2016;138(7):1565–1576.
- McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Urol. 2005;2(8):416–422.
- Gavert N, Sheffer M, Raveh S, et al. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res. 2007;67(16):7703–7712.
- Gavert N, Conacci-Sorrell M, Gast D, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168(4):633–642.
- Gavert N, Vivanti A, Hazin J, et al. L1-mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol Cancer Res. 2011;9(1):14–24.
- Gavert N, Ben-Shmuel A, Lemmon V, et al. Nuclear factor-kappaB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. J Cell Sci. 2010;123(12):2135–2143.
- Er EE, Valiente M, Ganesh K, et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol. 2018;20(8):966–978.
- Obenauf AC, Massague J. Surviving at a distance: organ specific metastasis. Trends Cancer. 2015;1(1):76–91.
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334.
- Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529(7586):316–325.
- Malladi S, Macalinao DG, Jin X, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165(1):45–60.
- Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807–817.
- Valiente M, Obenauf AC, Jin X, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–1016.
- Boland CR, Goel A. Prognostic subgroups among patients with stage II colon cancer. N Engl J Med. 2016;374(3):277–278.
- Marshall JL. Risk assessment in Stage II colorectal cancer. Oncology (Williston Park). 2010;24(1 Suppl 1):9–13.
- Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–3419.
- Gast D, Riedle S, Issa Y, et al. The cytoplasmic part of L1-CAM controls growth and gene expression in human tumors that is reversed by therapeutic antibodies. Oncogene. 2008;27(9):1281–1289.
- Arlt MJ, Novak-Hofer I, Gast D, et al. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006;66(2):936–943.
- Wolterink S, Moldenhauer G, Fogel M, et al. Therapeutic antibodies to human L1CAM: functional characterization and application in a mouse model for ovarian carcinoma. Cancer Res. 2010;70(6):2504–2515.
- Schafer H, Dieckmann C, Korniienko O, et al. Combined treatment of L1CAM antibodies and cytostatic drugs improve the therapeutic response of pancreatic and ovarian carcinoma. Cancer Lett. 2012;319:66–82.
