Abstract
Background: The Danish Cancer Registry (DCR) and the Danish Colorectal Cancer Group (DCCG) database are population-based registries collecting information on Danish patients with colorectal cancer (CRC). DCR registers all patients with incident CRC whereas DCCG records patients with first time CRC. The registries use different inclusion criteria. The consequencenses of this are unknown and not previously evaluated. The aim of this study was to examine the agreement between patients registered in DCR and DCCG and to evaluate its influence on estimated survival and mortality.
Material and methods: Patients registered in DCR and DCCG with CRC in 2014–2015 were included. Because of different inclusion criteria, DCCG’s inclusion criteria were applied to DCR. Descriptive statistics were used for comparisons. One-year relative survival (1-year RS) was calculated, and the Cox proportional hazard model used for calculating 1-year mortality rate ratios (1-year MRR).
Results: In 2014–2015, DCR registered 9678 Danish residents with CRC that fulfilled DCCG’s inclusion criteria, while DCCG registered 10,312 Danish residents with CRC. Allowing ±180 days between dates of diagnosis, 10,688 patients were registered with CRC in the merger of the two registries. Of these, 86% were included in both registers, 4% only in DCR, and 10% only in DCCG. No difference was found in 1-year RS between patients in DCR 86% (95% CI: 85–87) and DCCG 85% (95% CI: 84–86). However, patients registered in DCCG had a 1-year MRR of 1.09 (95% CI: 1.01–1.17) compared to DCR.
Conclusion: An agreement of 86% of patients was found between the two registries. The discrepancy did not influence 1-year RS. DCCG registered more patients than DCR, and 1-year MRR of patients in DCCG was increased compared to patients in DCR. Regular linkage of the registries is recommended to improve data quality of both registries.
Background
The Danish Cancer Registry (DCR) is a population-based registry collecting information on patients with incident cancer in Denmark since 1943. The primary purpose of the registry is to monitor the incidence and survival of all patients with cancer.
The Danish Colorectal Cancer Group (DCCG.dk) database (named DCCG in the present paper) is a population-based, clinical quality database collecting data since 2001 on patients with primary colorectal cancer (CRC) – the third most common cancer in Denmark [Citation1]. The primary aim of the database is to improve the prognosis of Danish CRC patients by monitoring the quality of the diagnostic workup and treatment of patients.
The two registries use different criteria of inclusion and coding practices of patients with CRC. The consequencenses here of are unknown and have not previously been evaluated.
DCR has previously been validated against other Danish clinical quality databases including databases for breast cancer and lung cancer, respectively, where the proportion of patients registered in both registries was 86%–92% [Citation2]. In Norway, the proportion of patients registered with CRC in both the Cancer Registry of Norway and the Norwegian Patient Registry has been evaluated [Citation3]. It was found that the proportion registered in both registries with colon cancer was 81%, and for patients with cancer at the rectosigmoid junction, rectum, or anus was 82%. Whether these differences had any impact on the calculated survival was not investigated.
Possible differences found in population-based registries should be taken into account when data from the various registries are used by epidemiologists, clinicians, and administrators for estimations of incidence and survival of the differenct cancer diseases. In Denmark, such estimations are used in order to improve prognosis and plan capacity for diagnostics, treatment, and follow-up for instance as done by the introduction of the Danish Cancer Pathways [Citation4]. For CRC, epidemiological and clinical Danish studies are based on data from both DCR and DCCG. Long-term survival of Danish CRC patients has improved over the last decades and the gap to other Scandinavian countries has decreased [Citation5]. To maintain this positive development, it is crucial to have valid and up-dated data of Danish CRC patients. However, DCR and DCCG calculate and report mortality and survival using different methods which makes comparisons difficult.
The aim of this study was therefore to examine the agreement between Danish CRC patients registered in DCR and DCCG and evaluate its influence on estimates of survival and mortality. Further, we looked into the registries to find possible reasons for identified differences making it possible to take such differences into consideration in future studies.
Material and methods
Since 1968 every citizen in Denmark has been assigned a unique Civil Personal Registration (CPR) number which is used in all national registers, making linkage of data from different registries possible [Citation6].
Data sources
The Danish Civil Registration System (CRS) includes, among others, information on gender, place of residence, emigration, immigration, disappearance, and vital status [Citation6]. CRS is used for identification of Danish citizens and non-citizens with a temporary stay in Denmark in all sectors of public administration including the health care system.
The Danish National Patient Registry (NPR) records information on all admissions to Danish hospitals from 1977 including information on diagnosis and treatment procedures and is the primary basis for monitoring of activities and thus for allocation of resources to the public Danish hospitals [Citation7].
The Danish Pathology Register (DPR) collects information from all departments of pathology including all text reports and pathological diagnoses. Diagnoses are coded according to a Danish variant of the SNOMED classification and include as a minimum topography and morphology codes [Citation8].
Data from NPR and DPR were used in the present study to validate the registrations in DCR and DCCG. The data in NPR and DPR were not validated.
DCR was established in 1943, and since then all incident cancer patients have been registered. Data are extracted from NPR and the Danish Register of Causes of Death. Furthermore, DCR is linked to DPR for further information about histology of identified cases. DCR includes CRC patients with all types of histology, patients without information on histology, and patients with metachronous CRC. The registry has information about patient and tumor characteristics. Tumor characteristics included are, that is, ICD-7/ICD-10 diagnoses, morphology, topography, and date of diagnosis [Citation9,Citation10]. After a modernization in 2004, 80%–90% of all cancer patients are registered automatically using automated algorithms. The rest is assessed manually after contact with the relevant clinical department. The coding manual is built on recommendations from the International Association of Cancer Registries and the European Network of Cancer Registries with some Danish exceptions. For CRC, DCR includes all patients with the diagnoses C18-C20.9 when registered as incident cases of cancer. The date of diagnosis in DCR is defined as the admission date for the first contact during which the diagnosis was confirmed. If the date of diagnosis not can be identified, DCR considers the registration as incomplete and the case is not registered.
DCCG was established in 2001 and includes all Danish citizens aged at least 18 years with a first-time diagnosis of CRC. The data sources are data from DPR and NPR, supplied by notifications on diagnostics, treatment, among others, from the surgical departments at public Danish hospitals [Citation11]. Private hospitals in Denmark are not involved in management of patients with CRC. In DCCG, the date of diagnosis is defined as the date of the biopsy result, or the date of operation in case of no preoperative biopsy, or if neither of these were performed, the date when the patient was informed about the CRC diagnosis. In Denmark, the great majority of patients are in fact investigated by a biopsy before the definitive treatment plan is decided upon. But in a few cases, the morphology of the tumor is unknown. Patients with the following types of histology of CRC are included: adenocarcinoma, poorly differentiated adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma, medullary carcinoma, and undifferentiated carcinoma. Patients with metachronous or recurrent CRC are not included. From 2010 and onward, patients with cancer of the appendix (C18.1) have not been included.
DCR and DCCG use different coding practices of morphology which may result in different registrations of an individual patient: If a patient is registered in DPR with more than one code of morphology from the same tumor, DCR’s algorithm chooses automatically the highest numerical code value of morphology in the International Classification of Diseases for Oncology (ICD-O). On the contrary in DCCG, the coded morphology is selected by a pathologist. Thus, if for instance, a tumor is found to be a neuroendocrine carcinoma (ICD-O code M82463) on a biopsy, but later is diagnosed as an adenocarcinoma (ICD-O code M81403) with a neuroendocrine component in the resection specimen, DCR would register the tumor as a neuroendocrine carcinoma, whereas DCCG would register the tumor as an adenocarcinoma, according to the WHO classification of tumors of the gastrointestinal tract.
The different coding practices of the date of diagnosis of the two registries may also result in patients being registered in different periods (i.e., before or after a particular study period). For the majority of patients, DCCG uses the date of biopsy result as the date of diagnosis, while DCR uses the date of admission for the first contact where the diagnosis was confirmed. Consequently, an individual patient registered in DCCG can have a different date of diagnosis than in DCR.
Patient cohort
All patients registered with CRC in DCR and DCCG from 1 January 2014 through 31 December 2015 were included in the study. Comparison of registered patients in the registries was done through linkage using the unique CPR number.
Patients diagnosed with a metachronous CRC were excluded, as were patients who were not residents in Denmark.
Statistical methods
Due to the different definitions of dates of diagnosis in DCR and DCCG, registrations of individual patients were considered identical when registered in both registries with up to 180 days between the two dates. Individual patients registered in both registries, but with more than 180 days between the dates of diagnosis, were considered non-identical and excluded from the present study as we assumed these patients to suffer from metachronous or recurrent CRC.
Because of the more strict inclusion criteria used by DCCG when compared to DCR regarding localization and histology of tumors, as described above, registered patients in the registries were compared both before and after application of the inclusion criteria of DCCG on data from DCR.
Agreement between patients found in the two registers was determined as patients with the same CPR number, and less than 180 days between dates of diagnosis in DCR and DCCG. Data are presented as proportions.
The term ‘intersection’ indicates patients in the merger of the two registers found in both registers, whereas the term ‘set difference’ indicates patients found only in one of the registers [Citation12].
All patients were followed from the date of diagnosis for 1 year and 3 years, until date of death, emigration according to CRS whichever came first.
DCR and DCCG use different models for estimating survival and mortality in their annual reports. DCR calculates 1-year relative survival (RS), while DCCG calculates Cox proportional hazards models. Therefore, in the present study, both methods were applied.
One-year age-standardized relative survival (1-year RS) was calculated using the International Cancer Survival Standard as used in the Nordic Cancer Survival Study [Citation13]. Relative survival was defined as the observed survival divided by the expected survival, that is, only deaths due to CRC were included. The observed survival was estimated using the actuarial method and the expected survival by the Ederer II method [Citation14]. The expected survival was estimated from male and female population mortality rates stratified by age and calendar time in 1-year intervals, respectively.
For the Cox Proportional Hazard models 1-year mortality (1-year MRR) indicating mortality from all reasons was calculated using time since the date of diagnosis as the underlying timescale, while gender and age at time of diagnosis were included as strata. The assumption of proportional hazards was evaluated, and no variables were considered to violate the assumption.
Kaplan-Meier curves showing the proportion of patients alive as a function of time were used to further visualize the survival in the different groups.
SAS® statistical software (release 9.4, SAS Institute, Inc., Cary, NC, USA) was used for all statistical analyses.
Results
All patients with CRC registered in DCR and DCCG
In the period 2014–2015, a total of 11,374 Danish residents with CRC were identified in the entire DCR (DCRtotal). In the same period, DCCG registered 10,312 patients with CRC (DCCGtotal). When combining DCRtotal and DCCGtotal 11,747 patients were identified. Of these patients, 66 were registered with more than 180 days between the dates of diagnosis in the two registries and were excluded (data not shown), leaving a total of 11,681 patients in the merger of DCRtotal and DCCGtotal (Data not shown).
For the combination of DCRtotal and DCCGtotal, the agreement between patients in the two registries, that is, patients found in the intersection of the two registries, was 85% (n = 9977; ). Eleven % (n = 1331) were registered in the set difference of DCRtotal and not in DCCGtotal, and 3% (n = 373) were registered in the set difference of DCCGtotal and not in DCRtotal.
Table 1. Registered Danish patients with CRC in 2014–2015.
Application of the inclusion criteria of DCCG on data in DCR
To further evaluate the agreement between DCRtotal and DCCGtotal, the inclusion criteria of DCCG were applied to patients in DCRtotal, that is, the following patients were excluded from the 11,374 patients in DCRtotal: Patients with CRC with other types of histology than included in DCCG, patients with metachronous CRC, patients with appendix cancer, and patients less than 18 years old at time of diagnosis (of which none were found). After this exclusion, 9678 patients who fulfilled the inclusion criteria of DCCG were identified in DCR and named DCRDCCG ().
Figure 1. Definition of the patient cohort. DCR: Danish Cancer Registry; DCCG: Danish Colorectal Cancer Group; CRC: colorectal cancer. 1Patients registered in DCCG in 2014–2015 with a Danish residence at the time of diagnosis. 2Number of tumors (C18-C20.9) of patients registered in DCR in 2014–2015 with a Danish residence at the time of diagnosis. 3Patients registered in DCR that fulfill the inclusion criteria of DCCG.
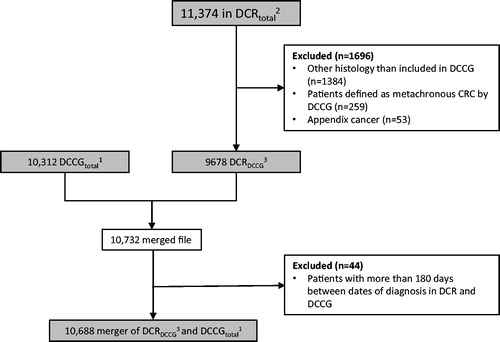
When merging DCRDCCG and DCCGtotal, 10,732 individual patients were identified. Of these 44 patients were registered with more than 180 days between the dates of diagnosis in the two registries and were excluded, leaving a total of 10,688 patients in the merger of DCRDCCG and DCCGtotal ().
In the merger of DCRDCCG and DCCGtotal the agreement between the two registries was 86% (n = 9214; ), while 4% (n = 420) of the patients were registered in the set difference of DCRDCCG and not in DCCGtotal, and 10% (n = 1054) were registered in the set difference of DCCGtotal and not in DCRDCCG.
shows that of the 420 patients only found in the set difference of DCRDCCG, and not in DCCGtotal, 267 had a diagnosis in DPR that fulfilled the inclusion criteria of DCCG, and furthermore, seven patients were registered in DCCG before the study period. The remaining 146 patients were identified as patients registered in DPR with a tumor with a morphology code that does not fulfill the inclusion criteria of DCCG (n = 111), patients with identical dates of diagnosis and death that is, possible cases of ‘Death Certificate Only (DCO)’ (n = 11), various explantions as decribed in (n = 17), or with unknown explanation (n = 7).
Table 2. Patients ony found in the set difference of DCRDCCG and not in DCCGtotal in 2014 and 2015 from the merger of DCRDCCG and DCCGtotal.
shows that of the 1054 patients only found in the set difference of DCCGtotal and not in DCRDCCG, 122 had a CRC diagnosis in NPR that fulfilled the inclusion criteria in DCR. Furthermore, 151 patients had an incomplete registration of CRC in NPR not considered sufficient by DCR. Additionally, 360 patients in the set difference of DCCGtotal had a registration of CRC in DCR in the study period, but with a different morphology code than in DCCG. The number of patients registered in DCCGtotal in year(s) before or after the study period was 377 (n = 371 + 6, respectively). The remaining 44 patients were identified as patients with possible DCOs (n = 5), another type of cancer than CRC (n = 1) or with unknown explanation (n = 38). In total 122 + 5 = 127 patients fulfilled the inclusion criteria of DCR.
Table 3. Patients with CRC found only in the set difference of DCCGtotal and not in DCRDCCG in 2014 and 2015 from the merger of DCRDCCG and DCCGtotal.
The agreement between the two registries of patients in the merger of DCRDCCG and DCCGtotal was 86% (). For the two set differences of DCRDCCG and DCCGtotal, the different determinations of dates of diagnosis (n = 384 = 7 + 377; and ) and morphology codes (n = 360; ) explained 50% of the patients found in the respective two set differences. The last 50% are patients who were only registered in one of the registers.
A total of 540 (=267; +122 + 151; ) patients fulfilled the criteria of both registries, but were only registered in one of them and found in the respective set differences. If these 540 patients plus the 360 patients in the set difference of DCCGtotal registered with a different morphology code than in DCR () were included in the intersection, the agreement between the registries would increase to 95%. The remaining 5% consist of DCOs and unexplained cases.
Relative survival
For patients in DCRtotal the 1-year RS was 84% (95% CI: 84–85) which was slightly, but not significantly, lower than for patients in DCRDCCG and DCCGtotal who had 1-year RS at 86% (95% CI: 85–87) and 85% (95% CI: 84–86), respectively (). No difference was observed in 1-year RS when using the different dates of diagnosis as entry dates for patients in the intersection of DCRDCCG and DCCGtotal. However, lower 1-year RS were found for patients in both set differences of DCRDCCG and of DCCGtotal, namely 71% (95% CI: 67–77) and 71% (95% CI: 68–74), respectively () than for all patients in DCRDCCG and DCCGtotal. Calculations of 3-year RS demonstrated very similar results (Data shown in Supplementary Appendix).
Table 4. One-year Relative Survival (1-year RS) of Danish patients diagnosed with CRC in 2014 and 2015.
Cox proportional hazard models
Significantly lower mortalities were found for patients in DCRDCCG and DCCGtotal compared to patients in DCRtotal, when using Cox proportional hazard models (). Patients in DCCGtotal were found to have a significantly increased mortality rate ratio (MRR) at 1.09 (95% CI: 1.01–1.17) compared with patients in DCRDCCG. No difference was observed in MRR when using the different dates of diagnosis as entry dates for patients in the intersection of DCRDCCG and DCCGtotal. Mortality was non-significantly increased for patients in DCRDCCG, 1-year MRR 1.06 (95% CI: 0.98–1.14), while for patients in DCCGtotal mortality was significantly increased 1-year MRR 1.14 (95% CI: 1.06–1.22), when compared to patients in the intersection of DCRDCCG and DCCGtotal. Furthermore, also significantly increased MMRs were found for patients in the set differences of DCRDCCG and of DCCGtotal when compared to patients in the intersection of DCRDCCG and DCCGtotal, MRR: 2.33 (95% CI: 1.95–2.78) and MRR 2.39 (95% CI: 2.13–2.69), respectively. As observed for survival, estimations of 3-year MRR gave similar results as 1-year MMR (data shown in Supplementary Appendix).
Table 5. Cox Proportional Hazard models for Danish patients diagnosed with CRC in 2014 and 2015.
Kaplan-Meier curves
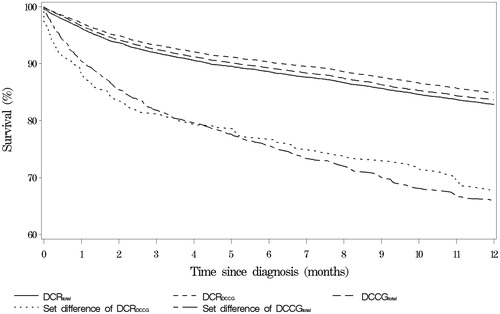
Kaplan-Meier curves show that survival of patients in DCRtotal, in DCRDCCG, and in DCCGtotal were very similar, whereas survival for patients in the set differences of DCRDCCG and of DCCGtotal was lower than for patients in DCRtotal, DCRDCCG, and DCCGtotal ().
Figure 2. Kaplan-Meier curves for selected groups. The fractional survival of patients with CRC as a function of time since diagnosis. DCRtotal: all CRC tumors registered in the Danish Cancer Registry; DCRDCCG: patients registered in the Danish Cancer Registry that fulfill the inclusion criteria of DCCG; DCCGtotal: patients registered in DCCG with residence in Denmark at time of diagnosis. Set difference of DCCGtotal: patients registered in DCCG and not in DCR.
In the Supplementary Appendix, all the analyses mentioned above are shown stratified for gender and cancer site (colon/rectum). Similar patterns were found irrespective of gender and cancer site. Furthermore, in the Supplementary Appendix, tables corresponding to and are found for the set differences of DCRtotal and DCCGtotal. Also in the Supplementary Appendix data for relative survival and mortality rate ratios are shown for 3 years follow-up.
Discussion
In the present study, an agreement of 86% between patients registered with CRC in DCRDCCG (i.e., patients registrered in DCR that fulfill the inclusion criteria of DCCG) and DCCGtotal was observed. This finding is similar to previous studies where DCR was validated against other clinical quality databases containing data on other cancer diseases [Citation2]. Further, it was found that 50% of the discrepancies between DCRDCCG and DCCGtotal were due to differences in determinations of dates of diagnosis and different coding practices of morphology.
Both DCR and DCCG use the Danish Pathology Register to determine the morphology of the cancer. But the different inclusion criteria and coding algorithms may result in different registrations of the same incident as outlined in Methods, explaining why some patients are registered in DCR and not in DCCG.
Also the date of diagnosis is determined differently in the two registries, and even when an interval of up to 180 days between the two dates was allowed, some patients were registered in the other register either before or after the study period.
As expected, the different inclusion criteria of the registers contribute to the observed differences in numbers of registered patients. In the present study, it was found that if all patients as identified by the CPR number and registered with CRC, disregarding different registrations of morphology codes, possible incomplete registrations in NPR of CRC, and different dates of diagnosis were included in the intersection, the agreement between DCRDCCG and DCCGtotal would be 95%.
Each year a small group at ∼0.4% of patients in DCR is identified through death certificates only (DCO) [Citation15]. These patients may not have been in contact with a surgical department at a public Danish Hospital and are therefore neither registered in NPR nor in DCCG. In the present study only 11 patients (or 0.11%) found in DCR were possible cases of DCO.
We found that both DCRDCCG and DCCGtotal have registered patients in the set differences that seemed to fulfill the inclusion criteria of the other registry. Each year both registries identify patients that possibly should be included in the registry. Such patients are under investigation/examination with reminders being sent to the relevant departments. For a patient to be included in DCR and in DCCG, the patient must have a registration in NPR that fulfills the inclusion criteria or a notification from the surgical department. Thus, the clinical department must register the patient in two different sites which is not at present done automatically. DCCG reports the calculated completeness of data in their reports [Citation16]. However, no information is published from DCR about the number of patients who await further notification from the relevant departments.
DCR aims at including all incident cases of cancer located in the colon and rectum whether or not diagnostics or treatments have been perfomed, and irrespective of histological subtype or no histological classification, thus making estimations of the total incidence of CRC possible. However, the present study indicates that 122 patients may not have been registrered in DCRDCCG. The study does not include information on the remaining patients in DCRtotal who were not included in DCRDCCG. The inclusion criteria of DCCG with a restrictive definition of CRC regarding histology cause that not all cases of CRC are included, and furthermore, according to our findings, some cases fulfilling the criteria may not have been registered. It is therefore surprising that the number of patients (n = 10,312) in DCCGtotal is higher than the number of patients (n = 9678) in DCRDCCG-These discrepancies should be taken into account when evaluating the calculated incidence and mortality rates of CRC based on data from the registers.
Despite the different inclusion criteria of the two registries and the observed differences of registered patients, no significant differences were observed in the overall 1-year RS between patients in the registers. This finding is in accordance with previous publications from DCR and the so called Benchmarking II report based on data from DCCG [Citation17,Citation18]. However, for patients in the set differences of both registers decreased 1-year RS was observed when compared to the the total number of patients in the entire registries as supported by the Kaplan-Meier survival curves. These findings indicate that the mortality caused by CRC of patients in the two registries is the same despite the discrepancies found. On the contrary, in the Cox proportional hazard models increased 1- and 3-year mortality rates were found for patients in DCCGtotal compared to patients in DCRDCCG. This indicates that some patients registered in DCCG have a higher mortality from all causes than patients in DCRDCCG. Also patients in the two set differences, DCRDCCG and DCCGtotal had increased mortality rates when compared to patients in the intersection of these two groups.
The validity of the registrations of the two registries may be improved in different ways. One way could be to unify the definitions of the date of diagnosis and to agree upon a common coding practice of morphology, but also to agree upon whether metachronous and recurrent cases of CRC should be included or not. Further, the consequences of the strict inclusion criteria of DCCG may be disputed. The cases which are excluded from DCCG at present might be registered, grouped together, and published. Lastly, the registers might consider mutual information on identified new cases by regular linkage, thus minimizing the effort of further investigations and contacts to the clinical departments from the registries. Also such a regular linkage would minimize the demand to the clinical departments of notifying the same case in two different registers. An improvement in patient-completeness would support the primary purposes of both registries.
Conclusion
An agreement of 86% was found between patients with CRC registered in DCR and DCCG in the period 2014–2015 when the different criteria of inclusion were taken into account. Surprisingly, more patients were registered in DCCG than in DCR using the inclusion criteria of DCCG. Relative survival was very similar, but a significant higher mortality rate was observed among patients in DCCG compared to patients in DCR applying the inclusion criteria of DCCG when using a Cox proportional hazard model. Part of the discrepancies is due to different determinations of morphology codes, different determinations of date of diagnosis, and possible registration errors. If all patients were included in the intersection of the two registers, disregarding different dates of diagnosis and morphology codes, the agreement between the two registers would increase to 95%. Furthermore, the demand to clinical departments to notify the same patient twice may contribute to the discrepancies.
| Abbreviations | ||
| CPR | = | Civil Personal Registration |
| CRC | = | Colorectal cancer |
| CRS | = | The Danish Civil Registration System |
| DCCG | = | The Danish Colorectal Cancer Group |
| DCCGtotal | = | Danish residents registered in DCCG |
| DCR | = | The Danish Cancer Registry |
| DCRDCCG | = | Danish residents registered in DCR with CRC and fulfilling the inclusion criteria of DCCG |
| DCRtotal | = | Danish residents registered in DCR with CRC |
| DPR | = | The Danish Pathology Register |
| NPR | = | The Danish National Patient Registry |
| 1-MRR | = | Mortality Rate Ratio for 1 year |
| 1-year RS | = | One-year relative survival |
Supplemental Material
Download MS Word (49.2 KB)Disclosure statement
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Danish Cancer Society [Internet]. The most common cancers (in Danish). Available from: https://www.cancer.dk/hjaelp-viden/fakta-om-kraeft/kraeft-i-tal/de-hyppigste-kraeftformer/
- Statens Serum Institute [Internet]. Validation of the Danish Cancer Registry and selected clinical databases (In Danish). 2012. Available from: http://sundhedsdatastyrelsen.dk/-/media/sds/filer/registre-og-services/nationale-sundhedsregistre/sygedomme-laegemidler-og-behandlinger/cancerregisteret/valideringsrapport-cancerregisteret.pdf?la=da
- Bakken IJ, Gystad SO, Christensen OO, et al. Comparison of data from the Norwegian Patient Register and the Cancer Registry of Norway. Tidsskriftet. 2012;132(11):1336–1340.
- Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians—A national Danish project. Health Policy. 2012;105(1):65–70.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7):22–25.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7):30–33.
- Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39(7):72–74.
- Pukkala E, Engholm G, Hojsgaard Schmidt LK, et al. Nordic Cancer Registries – an overview of their procedures and data comparability. Acta Oncol. 2018;57(4):440–455.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7):42–45.
- Ingeholm P, Gogenur I, Iversen LH. Danish Colorectal Cancer Group Database. CLEP. 2016;8:465–468.
- Shen S, Vereshchagin NK, Shen A. Basic Set Theory. American Mathematical Society; 2002.
- Engholm G, Gislum M, Bray F, et al. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Material and methods. Acta Oncol. 2010;49(5):545–560.
- Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Statist Med. 2004;23(1):51–64.
- Health Data Board [Internet]. Incident cancers in Denmark (In Danish). 2014. Available from: http://sundhedsdatastyrelsen.dk/-/media/sds/filer/registre-og-services/nationale-sundhedsregistre/sygedomme-laegemidler-og-behandlinger/cancerregisteret/valideringsrapport-cancerregisteret.pdf?la=da
- The Danish Colorectal Cancer Group [Internet]. The Annual Report (In Danish). 2014. Available from: http://sundhedsdatastyrelsen.dk/-/media/sds/filer/registre-og-services/nationale-sundhedsregistre/sygedomme-laegemidler-og-behandlinger/cancerregisteret/valideringsrapport-cancerregisteret.pdf?la=da
- Health Data Board [Internet]. Survival of patients with cancer in Denmark (In Danish). Available from: http://www.esundhed.dk/sundhedsregistre/CAR/CAR03/Sider/Tabel.aspx
- DMCG.dk [Internet]. Benchmarking II Consortium: Uddybende rapport om canceroverlevelse i Danmark 1995–2014 (In Danish). 2017. Available from: http://dmcg.dk/dmcgdk-benchmarking-consortium/
