Abstract
Background: We evaluated efficacy and toxicity of 68Ga-PSMA-Positron Emission Tomography/Computed Tomography (PET/CT)-directed stereotactic body radiotherapy and image-guided radiotherapy (SBRT/IGRT) for oligometastases of prostate cancer recurrences after previous surgery.
Methods: Nineteen patients were analyzed within a prospective PET-registry study (064/2013BO1) and retrospectively analyzed (807/2017BO2) fulfilling the following inclusion criteria: biochemical recurrence after radical prostatectomy, ≤five 68Ga-PSMA-PET/CT positive lesions. Biochemical control was evaluated with EORTC (European Organization for Research and Treatment of Cancer)- and Phenix-definitions. Toxicity was scored according to CTCAE-criteria v. 4.03.
Results: A total of 38 oligometastases (19 patients, 2 with re-treatment) were treated with SBRT/IGRT from October 2014 to July 2017. 68Ga-PSMA-PET/CT-positive lesions were detected on average 39 months (5–139) after prostatectomy (pT2b-3b pN0-1 cM0). Mean PSA (Prostate-specific antigen)-level at time of imaging reached 2.2 ng/mL (range 0.2–10.1). PET/CT-positive lesions were treated with different fractionation schedules reaching biological equivalent doses (BED) of 116.7–230.0 Gy. Concomitant androgen deprivation therapy (ADT) was given in seven patients. After a median follow-up of 17 months (4–42) all patients were alive. Estimated 1-year PSA- control (n = 19) reached 80.8% (Phenix) and 67.5% (EORTC). A PSA-decline (≥50%) was detected in 16/19 patients after radiotherapy. Higher graded G3+-acute toxicity did not occur. Temporary late G3-proctitis was detected in one patient.
Conclusions: Reaching of nadir ≤0.1 or 0.2 ng/mL was associated by improved DMFS (distant metastases free survival) and could serve as a surrogate endpoint for RT of oligometastases after initial prostatectomy. Short term effects of 68Ga-PSMA-PET/CT-based ablative radiotherapy for oligometastases demonstrated an acceptable toxicity profile and favorable biochemical response.
Introduction
Stage IV disease of prostate cancer is defined as node positive or metastatic disease (cN1/cM1). According to current guidelines [Citation1–3] androgen deprivation therapy (ADT) is the standard of care. However, recent investigations suggest a superior survival for patients with oligometastases. This is a transitional state which was defined by Helman et al. as limited disease in a single or a small number of organs [Citation4]. Tree et al. hypothesized that treatment of oligometastases could prolong PFS (progression free survival) by 2–4 years in 20–40% of patients with different cancer sites including prostate carcinoma [Citation5]. Currently, there is no consensus on the number of nodal or bone lesions defining the “oligo”-stage [Citation6]. According to Bluemel et al., oligometastases were defined as ≤ five lesions [Citation7].
Oligorecurrence is defined as appearance of metachronous oligometastases after local control of the primary tumor [Citation8]. Feasibility data on metastasis-directed-treatment for oligorecurrences without ADT suggest low toxicity rates with SBRT (stereotactic body radiotherapy) [Citation9–12]. Prospective data with choline or sodium fluorid PET (Positron Emission Tomography) [Citation9,Citation10] demonstrate prolongation of PFS and a delay of the need of ADT compared to observation alone [Citation13]. However, in the Prostate-Specific Membrane Antigen-Positron Emission Tomography (PSMA-PET)-era Kneebone et al. demonstrated conflicting results with distant recurrences in most patients within 15 months of follow-up [Citation11].
On account of this controversy regarding the impact of local therapy in limited stage IV disease we decided to retrospectively evaluate outcome of patients receiving ablative RT (radiotherapy) for ≤ five macroscopic oligometastases (rcN1/cM1. The aim of this study was to investigate the efficacy and toxicity of image-guided radiotherapy (SBRT/IGRT) for oligometastatic patients with biochemical recurrence after previous surgery. We focused on 68Ga-PSMA-PET/CT (Prostate-Specific Membrane Antigen-Positron Emission Tomography/Computed Tomography)-based treatment due to superiority of the tracer compared to 11C-Choline [Citation14].
Material and methods
Patients
Patients were analyzed within a prospective PET-registry study (064/2013BO1). The data analysis was approved by the ethics committee of the Medical Faculty (807/2017BO2). We analyzed patients fulfilling the following criteria: biochemical recurrence (BCR) (two consecutive rises in PSA-level with the second rising PSA (Prostate-specific antigen)-level >0.1 ng/mL, or 3 consecutive rises) [Citation15] after radical prostatectomy (RP) (prior salvage RT allowed), ≤five 68Ga-PSMA-PET/CT positive lesions [Citation7] and ablative treatment, further details are given in Supplementary Table 1.
68Ga-PSMA-PET/CT
68Ga-PSMA-PET/CT scans (Biograph mCT Siemens Healthcare, Knoxville TN) were acquired after intravenous administration of 68Ga-PSMA and combined with computed tomography. Examination field comprised subcranial region to mid-thigh. Syntheses of radiopharmaceuticals and details of PET/CT acquisition were described before [Citation14].
Treatment
Radiotherapy of every PET/CT-positive lesion was planned with the intent receiving local control at the irradiated region. Ablative treatment was defined as minimal Biological Equivalent Dose (BED) values (α/β = 1.5 Gy) of 116.7 Gy. RT was planned based on one to three planning CTs in supine position (slice thickness: 2–3 mm) in order to estimate the organ at risk motions and volume changes. All lesions were contoured as GTV (gross tumor volume). Clinical target volumes (CTV) and organs at risk (OAR) were contoured in each CT-dataset by two radiation oncologists (ACM, FP) with assistance of a specialized radiologist for prostate cancer (CP). For standard fractionated intensity modulated RT (IMRT) (usually N1) we used a 6–7 mm margin (elective pelvis (7 mm), nodal boost (6 mm)) while hypofractionated SBRT/IGRT was delivered with a 2–6 mm safety margin encompassing the GTV. IMRT treatment plans were generated with the software package Hyperion® (University of Tübingen, Tübingen, Germany) employing a Monte-Carlo dose engine. IMRT was delivered with a 6/15 MV linear accelerator (Elekta Synergy S/Elekta Versa HD, Elekta Oncology Systems®, Crawley, UK) equipped with a 4–5 mm multileaf collimator in volumetric-modulated arc therapy (VMAT) as image-guided radiotherapy (IGRT) with daily conebeam CT (CBCT).
ADT was discussed with patients being node-positive in accordance with national guidelines. Acute toxicity was documented weekly during radiotherapy and 3 months thereafter according to RTOG (Radiation Therapy Oncology Group) classification [Citation16] and Common Toxicity Criteria of Adverse Events (CTCAE) classification version 4.03 [Citation1,Citation17]. The follow-up included medical history, clinical examination, PSA-level and toxicity assessment. Biochemical no evidence of disease (bNED) was defined using two recurrence definitions (+0.2 ng/mL (EORTC) [Citation18] and (nadir + 2 ng/mL (Phenix)) [Citation19]. Local control was defined either as bNED or as PET/CT-negative imaging results at treated localization. Distant metastasis-free survival was defined as time until occurrence of M1-disease. Time to any secondary treatment was defined as time until initiation of local or systemic treatment. Time without systemic treatment (TWIST) was defined as period until start of systemic therapy.
Statistical analysis
Descriptive statistical analysis was performed with Microsoft Office 2013 (Software, Microsoft Corp., Redmond, USA). Color artwork was created with Canvas X (ACD Systems International Inc., British Columbia, Canada). Statistical analyses were conducted in SPSS 22 (IBM, Armonk, New York, USA).
Results
Patient characteristics
A total of 19 patients fulfilled the inclusion criteria in terms of a 68Ga-PSMA-PET/CT-directed radical radiotherapy of all lesions for BCR after surgery. Two patients received a second curatively intended SBRT/IGRT for one metastases after a 68Ga-PSMA-PET/CT-restaging for BCR. Taken together, 21 treatments (19 patients) of 34 oligometastases plus 4 concurrently detected local recurrences (LR) were performed (38 lesions).
Median age at first diagnosis was 64.4 years (range 52–76 years). Regarding pTNM at initial diagnosis, most patients presented with at least one high risk feature (pT3a: 5; pT3b: 7; pN1: 7; R1: 8). Salvage RT of the prostatic fossa (total dose: 66.0–74.0 Gy) received six patients 34.2 months (median; range 6–76 months) after RP. The pelvic nodes were included (pN1 with one positive lymph node) in two patients (2/6). Further details of pretreatment characteristics are given in .
Table 1. Initial treatment at prostate cancer diagnosis.
68Ga-PSMA-PET/CT-imaging was performed in all patients for BCR defined as at least two PSA rises reaching or exceeding 0.2 ng/mL. Mean PSA-level at time of imaging reached 2.2 ng/mL (range 0.2–10.1). A total of 38 68Ga-PSMA-positive lesions were found in PET/CT approximately 39.2 months (range 5–139 months) after RP. The affected regions (19 patients, 21 treatments) included eleven nodal recurrences (NR) (20 lesions) and ten bone oligometastases (14 lesions), . In addition, four LR were detected. Nodal oligometastases were mainly distributed at iliac and perirectal region. Bone metastases were predominately located at pelvic bones (7 lesions) or spine (6 lesions), . Since a clearly higher detection rate of 68Ga-PSMA PET/CT was reported by our group [Citation14] and many others, SBRT/IGRT have been based on PSMA findings taking into account that imaging is not verified by histology.
Table 2. Characteristic’s at time of 68Ga-PSMA-PET/CT.
Treatment schedules
Radiotherapy treatment aiming for local control was performed from October 2014 to July 2017. Thirty-eight 68Ga-PSMA-PET/CT-positive lesions were treated with different fractionation schedules, Supplementary Table 2. The calculated BED-values (α/β = 1.5 Gy) ranged from 116.7 to 230.0 Gy. In case of standard fractionation, total radiation doses ranged depending on localization between 50–72 Gy: LR received 66–74 Gy, NR 50–66 Gy and distant metastases 50–66 Gy. Hypofractionated treatment was applied to bone lesions with 40 Gy in 10 fractions. SBRT/IGRT was performed with three fractions each other day (3 × 10 Gy). Concomitant ADT was given in seven node-positive patients.
Response to treatment
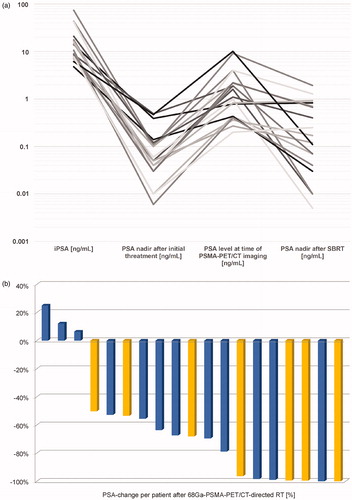
Course of PSA in ng/mL is presented from time of surgery, postoperatively at time of BCR before 68Ga-PSMA-PET/CT and after 68Ga-PSMA-PET/CT-directed treatment, . Each individual PSA-response after first 68Ga-PSMA-PET/CT-directed radiotherapy (n = 19) is presented as a waterfall plot, . A significant PSA decline (≥50%) was observed after first 68Ga-PSMA-PET/CT-directed treatment in 16/19 patients compared to PSA-level at time of 68Ga-PSMA-PET/CT- imaging.
Figure 1. Treatment related PSA-changes. (a) Logarithmic PSA course (ng/mL) is given for visualization of PSA-response (n = 19) at four timepoints: At initial diagnosis, after radical prostatectomy (+/− postoperative RT) at BCR before PET/CT examination and at nadir after (first) 68Ga-PSMA-PET/CT-directed treatment. (b) Waterfall plot of largest percentual change of PSA-level after first 68Ga-PSMA-PET/CT-directed treatment (n = 19; baseline: PSA-level at time of 68Ga-PSMA-PET/CT- imaging (ng/mL). Blue bars – without ADT, yellow bars – with ADT.
After a median follow-up of 17 months (range 4–42 months) calculated from start of radiotherapy all 19 patients were alive. Estimated biochemical control (n = 19) after 1/2 years reached 87.4%/79.4% (Phenix-definition, median 25 months) and 65.6%/41.0% (EORTC-definition, median 14 months), . Regarding 21 radiation treatments, estimated biochemical control after 1/2 years achieved 88.1%/80.8% (Phenix-definition, median 25 months) and 68.1%/34.0% (EORTC-definition, median 15 months).
Figure 2. Efficacy of 68Ga-PSMA-PET/CT-directed treatment. Biochemical control (n = 19) was calculated as Kaplan-Meier plot according to (a) Phenix-definition (nadir + 2 ng/mL) and (b) EORTC-definition (+0.2 ng/mL). (c) Lesion-specific (n = 38) local control (local recurrence, nodal recurrence, paraortic nodes and bone metastases) and (d) distant-metastasis-free survival (DMFS, n = 19) were demonstrated. Local control for all 38 lesions reached 94.7%.
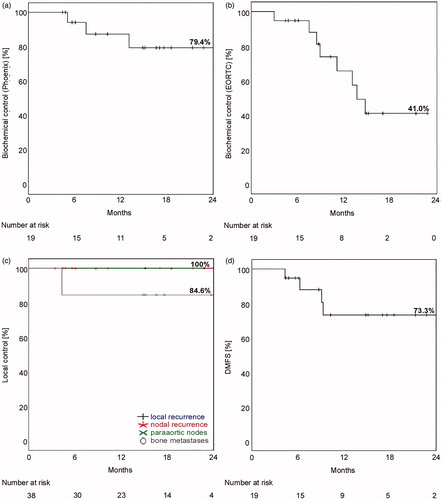
Local control reached 94.6%/95.0% for 19 patients/21 treatments. It was defined either as bNED or in case of BCR as negative imaging results at treated localization (PET/CT (n = 6) or bone scan (n = 1)). Lesion-specific local control reached 100% for N1-, M1a-disease or local recurrences. Local control for bone metastases was 84.6% related to progressive disease in one patient with two lesions, . Nine of 19 patients showed a new asymptomatic PSA increase. Restaging was performed with 68Ga-PSMA-PET/CT in 7/9 patients. One patient demonstrated multiple bone metastases in bone scan and therefore received no 68Ga-PSMA-PET/CT. Two other patients are already under PSA observation (PSA-level < 0.5 ng/mL) before imaging is initiated. 68Ga-PSMA-PET/CT-findings were always located in only one region i.e., bone metastases (n = 4), lymph node metastases (n = 2) and lung metastases (n = 1). Distant-metastasis-free survival after 68Ga-PSMA-PET/CT-directed RT reached after 1 and 2 years 73.3%, . An example of a representative radiation treatment plan for nodes is given in Supplementary Figure 1.
Further sub-analyses of evaluated endpoints were performed including ADT, PSA-nadir of 0.1 and 0.2 ng/mL. Significant results were only calculated for patients reaching nadir ≤ 0.1 ng/mL (p = .084) or nadir ≤ 0.2 ng/mL (p = .007) in regard to improved DMFS (distant metastases free survival) (2-year-DMFS: 100% vs. 22.2%).
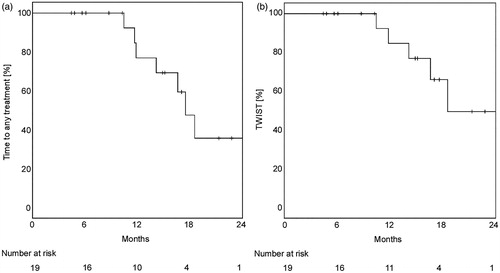
Treatment of second recurrences was performed as follows: Two patients received a second ablative IGRT for oligometastases after a 68Ga-PSMA-PET/CT-restaging for BCR (one combined with ADT), Supplementary Figure 2. Another patient received a salvage lymphadenectomy. The other four patients were treated with ADT alone. Time to any secondary treatment lasted for 18/21 months (mean/median). Time without systemic treatment (TWIST) reached in mean 24 months (median not reached), .
Figure 3. Time to next treatment. (a) Time to any treatment (n = 19) including ADT, IGRT and lymphadenectomy was calculated with the Kaplan-Meier method. (b) Time without initiation of systemic therapy (TWIST, n = 19) was demonstrated to visualize the efficacy of local second and third treatment after initial surgery to prolong initiation of systemic treatment.
Toxicity evaluation
No G3+ acute toxicity and no grade 4 late side effects were observed. According to CTCAE classification version 4.03, three patients developed acute gastrointestinal toxicity (G2) and one patient developed urogenital toxicity (G2). Intermittent (i.e., not persisting) late G2+-toxicity was observed in 2/19 patients (urogenital G2 and proctitis G3), Supplementary Table 3.
Discussion
We were able to show that 68Ga-PSMA-PET/CT-directed SBRT/IGRT of oligometastases was associated with an early PSA decline in over 80% of the patients. Using two definitions for biochemical endpoints of PSA response, we could demonstrate favorable biochemical control with a low toxicity profile. In addition, reaching of nadir nadir ≤0.1 or 0.2 ng/mL was associated by improved DMFS and could serve as a surrogate endpoint. Responsiveness of stage IV disease to local ablative norm-fractionated IGRT or hypofractionated SBRT was connected to excellent local control. Improved local control (95%) of first (n = 19) and second treatment (RT: 2, lymphadenectomy: 1) converted to prolonged TWIST. Use of ADT was mostly connected to treatment of nodal manifestations showing no significant difference in short-term follow-up (sub-analyses not shown). However, due to quite recent availability of 68Ga-PSMA-PET/CT, long-term follow-up has to be awaited.
Currently, there is no consensus on definition of BCR after salvage radiotherapy for macroscopic lesions. Therefore, we evaluated two endpoints i.e., EORTC-definition for salvage radiotherapy of biochemical recurrence for microscopic disease [Citation18] and as worst-case scenario the Phenix for macroscopic disease including patients receiving ADT [Citation19]. We decided not to use ASTRO (American Society for Radiotherapy and Oncology)-definition because we suspected fast PSA-rises in stage IV-disease leading to underestimation of relapses [Citation20]. Using both BCR-endpoints, might be useful to detect early progress (EORTC) to initiate re-staging or to begin treatment (Phenix) for significant disease. More than half of the patients did not progress within 17 months of follow-up after 68Ga-PSMA-PET/CT-directed RT. Regarding DMFS, this series compares well with other series in the same indication using SBRT ± ADT and 11C-choline PET/CT [Citation9]. Mean TWIST is in the same range like in the study of Ost et al. indicating efficacy of different fractionation schemes (BED > 116.7 Gy) [Citation13]. In line with others (NCT02264379) and the low number of in-field recurrences, this series further suggests that conventional fractionation like 50 Gy in 25 fractions (α/β = 1.5) might be sufficient for disease control of oligometastases. Furthermore, the surrogate endpoint nadir ≤0.1 or 0.2 ng/mL seemed to best predict PFS in this series.
Regarding detection rate of another PET-tracer, 68Ga-PSMA identified significantly higher number of PET-positive lesions compared to 11C-choline [Citation14]. Furthermore, 68Ga-PSMA-PET/CT demonstrated an improved cost efficacy with a number needed to image of 2 compared to 4 with 11C-choline PET/CT [Citation21]. Improved accuracy of 68Ga-PSMA was also substantiated by a previously performed meta-analysis [Citation22,Citation23] especially for BCR after RP with PSA-levels below 1.0 ng/mL [Citation24]. Respective sensitivity and specificity rates ranged from 61% to 84% [Citation24,Citation25] and from 82% to 100% [Citation25,Citation26]. Furthermore, 68Ga-PSMA detected mainly LR or NR at PSA-levels <1 ng/mL while at PSA-levels >1 ng/mL mainly distant metastases were observed [Citation26]. At very low PSA-levels ≤0.2 ng/mL, Gupta et al. reported a detection rate of 46% for BCR after surgery like in this series [Citation27]. However, uptake of 68Ga-PSMA is also observed in nonmalignant tissues (like ganglia) which has to be considered [Citation28]. In addition, regarding the pattern of recurrences i.e., mostly ex-field suggests that further increase of sensitivity of PET-tracers and -detectors might increase detection rate.
High local control rates were observed after PET/CT-directed radiotherapy in follow-up PET staging [Citation29] but optimal treatment of oligometastases is not well defined. It remains unclear whether addition of short- or long-term ADT is synergistic or initiates only palliative treatment from behind. However, in high risk disease, neo-/adjuvant ADT with RT improved survival and became standard of care [Citation30]. For stage IV defined by macroscopic pelvic nodes only (i.e., N1-disease), retrospective series showed a survival benefit of 10–20% within 5 years for combined use [Citation31–33]. In addition, ADT is recommended for 2–3 years in node-positive disease [Citation1–3]. Therefore, IGRT was combined with ADT in seven node-positive patients depending on individual preference. Regarding bone metastases, ongoing studies evaluate efficacy of hypofractionated or stereotactic RT without ADT and measure the time until next initiation of treatment [Citation34,Citation35]. Therefore, depending on expected treatment aim i.e., cure or even improved survival (N1) or prolongation of disease progression (M1b) different treatment concepts are currently under investigation including re-treatment of oligo-recurrences. Treatment of oligometastases is further supported by low toxicity rates with advanced imaging and treatment techniques [Citation13,Citation36,Citation37] as shown in this series. These considerations led to the recommendation of PSMA-PET/CT scans for BCR after surgery in case of expected therapeutic consequences i.e., treatment of oligometastases [Citation2]. Limitations of our study comprise a small patient cohort, no histologic verification of imaging due to ethical reasons and therefore no calculation of specificity or sensitivity.
We conclude that advanced 68Ga-PSMA-PET/CT-directed treatment is feasible with a mild toxicity profile and early PSA-response in >80% of patients. Data of this series show at least comparable PSA-responses compared to 11C-choline-PET/CT-directed treatment of oligometastases leading to excellent local control (95%) with different fractionation schedules. Reaching of nadir ≤ 0.1 or 0.2 ng/mL was associated by improved DMFS and could serve as a surrogate endpoint for RT of oligometastases after initial prostatectomy. To better define impact of tracer related accuracy and optimal patient selection longer follow-up and further prospective studies are awaited. Therefore, a retrospective multicentre analysis of the SBRT working group of DEGRO (Deutsche Gesellschaft für Radioonkologie) is planned.
Supplemental Material
Download (1.2 MB)Disclosure statement
The authors DZ and ACM declare scientific cooperation with Elekta®, Philips® und Siemens®. The other authors have no competing interests.
References
- NCCN Guidelines® & Clinical Resources [Internet]. Pennsylvania (US): National Comprehensive Cancer Network; 2019. [cited Aug 12]. Available from: www.nccn.org
- Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms Version 5.0 [German S3-Guideline] [Internet] Berlin (DE): Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF; 2019. [cited Aug 12]. Available from: www.leitlinienprogramm-onkologie.de
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–642.
- Hellman S, Weichselbaum RR. Oligometastases. JCO. 1995;13(1):8–10.
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14(1):e28–e37.
- Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73(2):178–211.
- Bluemel C, Linke F, Herrmann K, et al. Impact of (68)Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI Res. 2016;6(1):78.
- Palacios-Eito A, Bejar-Luque A, Rodriguez-Linan M, et al. Oligometastases in prostate cancer: ablative treatment. WJCO. 2019;10(2):38–51.
- Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69(1):9–12.
- Siva S, Bressel M, Murphy DG, et al. Stereotactic Abative Body Radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol. 2018;74(4):455–462.
- Kneebone A, Hruby G, Ainsworth H, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol. 2018;1(6):531–537.
- Incerti E, Gangemi V, Mapelli P, et al. 11C-Choline PET/CT based helical tomotherapy as treatment approach for bone metastases in recurrent prostate cancer patients. Curr Radiopharm. 2017;10(3):195–202.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. JCO. 2018;36(5):446–453.
- Schwenck J, Rempp H, Reischl G, et al. Comparison of (68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44(1):92–101.
- Ghadjar P, Hayoz S, Bernhard J, et al. Impact of dose intensified salvage radiation therapy on urinary continence recovery after radical prostatectomy: results of the randomized trial SAKK 09/10. Radiother Oncol. 2018;126(2):257–262.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. National Institutes of Health National Cancer Institute; 2010.
- Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366(9485):572.
- Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol. 2006;65(4):965–974.
- Consensus statement: guidelines for PSA following radiation therapy. American Society for therapeutic radiology and oncology consensus panel. Int J Radiat Oncol Biol Phys. 1997;37(5):1035–1041.
- Schwenck J, Olthof S, Pfannenberg C, et al. Intention to treat analysis of 68Ga-PSMA and 11C-choline PET/CT versus CT for prostate cancer recurrences after surgery. J Nucl Med. [cited 2019 Mar 8]. DOI:10.2967/jnumed.118.224543.
- Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926–937.
- Evangelista L, Briganti A, Fanti S, et al. New clinical indications for (18)F/(11)C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. Eur Urol. 2016;70(1):161–175.
- von Eyben FE, Picchio M, von Eyben R, et al. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2018;4:686–693.
- Herlemann A, Wenter V, Kretschmer A, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70(4):553–557.
- Budaus L, Leyh-Bannurah SR, Salomon G, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69(3):393–396.
- Gupta SK, Watson T, Denham J, et al. Prostate-specific membrane antigen positron emission tomography-computed tomography for prostate cancer: distribution of disease and implications for radiation therapy planning. Int J Radiat Oncol Biol Phys. 2017;99(3):701–709.
- Rischpler C, Beck TI, Okamoto S, et al. 68Ga-PSMA-HBED-CC uptake in cervical, celiac, and sacral ganglia as an important pitfall in prostate cancer PET imaging. J Nucl Med. 2018;59(9):1406–1411.
- Baumann R, Koncz M, Luetzen U, et al. Oligometastases in prostate cancer: metabolic response in follow-up PSMA-PET-CTs after hypofractionated IGRT. Strahlenther Onkol. 2018;194(4):318–324.
- Bolla M, Verry C, Long JA. High-risk prostate cancer: combination of high-dose, high-precision radiotherapy and androgen deprivation therapy. Curr Opin Urol. 2013;23(4):349–354.
- Da Pozzo LF, Cozzarini C, Briganti A, et al. Long-term follow-up of patients with prostate cancer and nodal metastases treated by pelvic lymphadenectomy and radical prostatectomy: the positive impact of adjuvant radiotherapy. Eur Urol. 2009;55(5):1003–1011.
- Abdollah F, Karnes RJ, Suardi N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. JCO. 2014;32(35):3939–3947.
- Lin CC, Gray PJ, Jemal A, et al. Response. J Natl Cancer Inst. 2015;107(9):djv200.
- Radiotherapy of the prostate and the Pelvic Lymph Nodes After Neoadjuvant Antihormonal Treatment (PLATIN). Clinical Trials. gov Identifier NCT01903408; 2018.
- Percutaneous High-dose Radiotherapy in Patients with Oligometastases of Prostate Carcinoma (Oli-P). ClinicalTrials.gov Identifier: NCT02264379; 2018.
- Muller AC, Lutjens J, Alber M, et al. Toxicity and outcome of pelvic IMRT for node-positive prostate cancer. Strahlenther Onkol. 2012;188(11):982–989.
- Henkenberens C, von Klot CA, Ross TL, et al. 68Ga-PSMA ligand PET/CT-based radiotherapy in locally recurrent and recurrent oligometastatic prostate cancer: early efficacy after primary therapy. Strahlenther Onkol. 2016;192(7):431–439.
