Introduction
Immune checkpoint inhibitors (ICI) have substantially improved outcomes for many advanced cancers. However, only a subset of patients respond to these therapies. There is currently no ideal biomarker to predict treatment benefit. Tumor expression of programed death ligand 1 (PD-L1) has been used in some settings, but its predictive value is not universally applicable across all cancers [Citation1]. More recently, tumor mutation burden (TMB) has been investigated as a promising biomarker for ICI benefit [Citation2–4].
Tobacco smoking is strongly implicated in tumor mutagenesis for non-small cell lung cancer (NSCLC), head and neck cancer (HNC), and urothelium cancer (UC). Molecular profiling of smoking-related NSCLC [Citation5] and HNC [Citation6] has shown that these are characterized by a greater mutation burden than tumors of never-smokers. However, in UC, no association has been reported between smoking and mutation type, spectrum, or frequency [Citation7].
In clinical practice, there is considerable interest in whether smoking status could be used to select patients for ICI therapy. Uncontrolled randomized trials of NSCLC have reported that smokers treated with ICI have more frequent tumor shrinkage than nonsmokers [Citation3,Citation8,Citation9]. Yet, in subgroup analyses of randomized trials, hazard ratios (HR) for progression free survival (PFS) inconsistently trended towards more benefit for ever-smokers or never-smokers [Citation10,Citation11]. No information on this association is available for other cancers. To address this question, we performed a systematic review and meta-analysis of randomized trials to compare the relative treatment benefit of ICI versus chemotherapy between ever-smokers and never-smokers.
Methods
We searched PubMed, MEDLINE and EMBASE from database inception until June 30, 2018, to retrieve phase 2 and 3 randomized studies comparing ICI alone versus chemotherapy alone in advanced cancers. The search terms were ‘Atezolizumab’ or ‘Durvalumab’ or ‘Ipilimumab’ or ‘Nivolumab’ or ‘Pembrolizumab’ or ‘Tremelimumab’ and ‘randomized’. Articles were limited to ‘English language’ and ‘Human’ studies. To identify unpublished trials and updated data for published trials, we hand-searched proceedings from international oncology conferences including the American Society of Clinical Oncology, the European Society of Medical Oncology, American Association for Cancer Research, and International Association for the Study of Lung Cancer from January 1, 2018 to June 30, 2018. Two investigators (KL and LK) independently performed the literature search, and extracted data from all studies. All discrepancies were resolved by consensus with all investigators.
Eligible trials studied ICI alone versus chemotherapy alone as non-curative intent treatment for cancer, and published the overall survival (OS) HR and 95% confidence intervals (CI) for all patients, as well as separately for ever-smokers and never-smokers. For each trial, KL and LK excerpted data on the study design, blinding, study phase, cancer type, treatments given, line of therapy, median follow-up time, primary endpoint and distribution of ever-smokers versus never-smokers. Former smokers and current smokers were analyzed as a group of ever-smokers. As smoking status may be associated with sex and age, we also examined treatment benefit for these subgroups to look for potential interaction in the study sample. Data on oncogenic mutations and PD-L1 expression were extracted as these are also known to potentially affect treatment effect of ICI. When there were duplicate publications for a trial, only the most recently reported data was analyzed. The Cochrane Risk of Bias tool was used to assess the methodological quality of studies for the OS outcome.
We used the fixed-effects inverse-variance-weighted method to pool trial data to estimate ICI benefit on OS. We used the I2 statistic and Cochran Q test to quantify heterogeneity across studies, and the latter to test for a smoking–treatment interaction.
Results
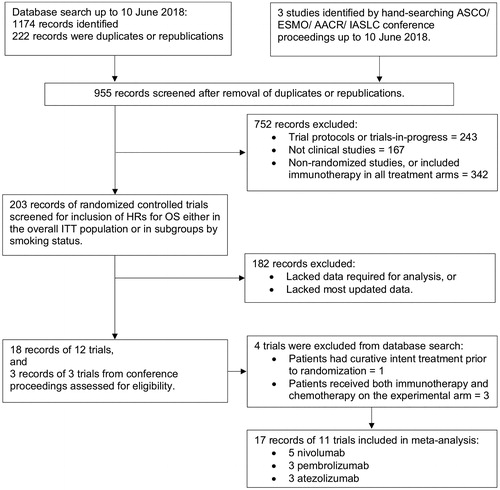
We identified 11 eligible trials (, Supplementary Table S1). All trials were open-label. Eight studies were assessed as low risk of bias [Citation10–21]. Methodological quality was unclear for three unpublished trials [Citation22–24].
Of 7014 advanced cancer patients, 4685 (67%) had NSCLC, 856 (12%) had squamous HNC, and 1473 (21%) had UC. Among NSCLC patients, 3882 (82%) had no oncogenic driver mutations, 750 (16%) had unknown mutation status, 103 (2%) had mutation of the epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genes. There were no mutation analyses for the trials of HNC and UC. Two NSCLC trials selected 1815 treatment-naïve patients with PD-L1 tumor proportion score of 1% or more [Citation13,Citation22]. All other trials included patients with any PD-L1 expression, who had received prior systemic therapy. Of the whole analyzed population, a total of 1526 (22%) were never-smokers and 5431 (77%) were current or ex-smokers. Fifty-seven patients (<1%) were excluded because smoking status or treatment effect was unknown.
Patients were randomized to receive ICI (N = 3654) or chemotherapy (N = 3360) (Supplementary Table S1). The ICI agents were atezolizumab (N = 1224), nivolumab (N = 1276), and pembrolizumab (N = 1154).
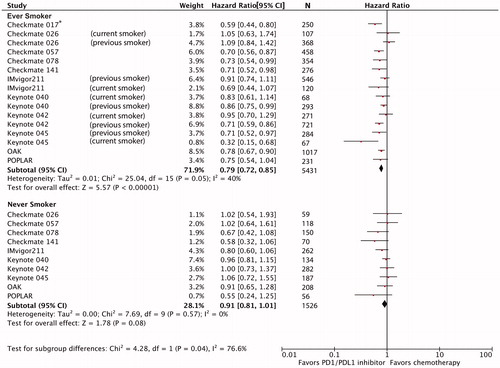
In the overall population, ICI improved OS over chemotherapy (pooled HR 0.81, 95%CI 0.76–0.81). There was a statistically significant interaction between treatment effectiveness and smoking status (ever-smokers [N = 5431]: HR 0.79, 95%CI 0.72–0.85; never-smokers [N = 1526]: HR 0.91, 95%CI 0.81–1.01; interaction-p = .04; ).
Figure 2. Forest plot of hazard ratios for overall survival comparing immune checkpoint inhibitors versus chemotherapy in ever-smokers and never-smokers patient subgroups. Hazard ratio for each trial is represented by the square and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamond represents the pooled overall effect size estimated using a fixed-effect meta-analysis. All statistical tests were two-sided. *Hazard ratio could not be estimated for never-smoker subgroup because there were only 17 patients in CHECKMATE 017 trial.
When the analysis was repeated in each tumor group, treatment benefit was numerically greater for ever-smokers than never-smokers for NSCLC (HR 0.78 versus 0.90) and UC (HR 0.70 versus 0.89), but not for HNC (HR 0.84 versus 0.82) (Supplementary Figures S1–3). The treatment–smoking interaction was not statistically significant within any of these tumor subgroups.
Nine trials reported treatment effectiveness by sex (males: N = 4031 [69.6%]; females: N = 1765 [30.5%]) [Citation10–14,Citation17–20,Citation22–24]. ICI benefit for OS did not differ significantly by sex (male HR 0.78, 95%CI 0.71–0.85; female HR 0.87, 95%CI 0.78–0.97; interaction-p = .13) (Supplementary Figure S4). Ten trials reported treatment effectiveness by age (<65 years: N = 3501 [57.6%]; ≥65 years: N = 2582 [42.4%]) [Citation10–15,Citation17–21,Citation23–25]. ICI benefit for OS also did not differ significantly by age (<65 years: HR 0.83, 95%CI 0.75–0.92; ≥65 years: HR 0.75, 95%CI 0.68–0.83; interaction-p = .19) (Supplementary Figure S5).
Discussion
In this meta-analysis, ICI therapy is associated with a 19% reduction in risk of death over chemotherapy for NSCLC, HNC, and UC. Across these three cancers, ICI therapy provided an additional 13.2% relative OS benefit over chemotherapy for ever-smokers compared to never-smokers (pooled HR 0.79 versus 0.91, interaction-p = .04). Within each tumor type, no statistically significant interaction between smoking status and ICI effectiveness was demonstrated.
Although ICI therapy demonstrates a higher relative OS advantage for ever-smokers, the estimate of treatment effect for never-smokers does not rule out a modest benefit, as the upper bound of the pooled confidence interval for OS HR just crossed unity. Despite pooling data from 11 trials involving 7014 patients, the statistical test of interaction was borderline (p = .04). Never-smokers only comprised a fifth of this analysis cohort, thus further study in larger samples of never-smokers will be important. Inconsistencies in the definition of smoking status between trials, unreliability of self-reporting, and failure to account for changes in tobacco usage over the entire trial period [Citation26] may also limit the study estimates. Alone, these findings do not support selecting patients for ICI therapy based on smoking status.
The validity of smoking history can be marred by recall bias and reporting bias. Thus, researchers have investigated objective biomarkers potentially linked to the effect of smoking. It is widely hypothesized that the benefit of ICI therapy for smokers is due to their greater tumor neoantigen load. Multiple studies have shown that TMB [Citation2,Citation3] may be a surrogate for overall neoantigen load. High plasma-based TMB reliably predicted improvement in progression-free survival (PFS) from atezolizumab over docetaxel in two second-line advanced NSCLC trials [Citation27]. High tissue-based TMB also predicted PFS improvement from nivolumab-ipilimumab over platinum chemotherapy in a large first-line advanced NSCLC trial [Citation28]. A retrospective cohort study of multiple different advanced cancers treated with ICI reported that higher TMB was associated with improved OS, but the TMB cut points associated with improved survival varied markedly between cancer types [Citation4]. The comparability of different TMB platforms, number and types of genes analyzed, and an appropriate cut point to classify patients as ‘high’ TMB still need to be addressed before this biomarker is suitable for routine use.
This study has several strengths. We used trial data to provide robust randomized comparisons and examined OS as the most clinically relevant endpoint. To date, this is the largest study to assess the interaction between smoking and ICI effectiveness, and the first meta-analysis to examine other tobacco-implicated cancers beyond NSCLC. This study also has several limitations. None of the trials provided clear definitions for smoking exposure or reported changes in smoking status during the study. We were unable to compare the HR PFS or response rates between ever-smokers and never-smokers due to paucity of published data. Prior studies have reported a greater OS benefit for ICI in men than in women [Citation29], and for younger versus older patients [Citation30]. Our prior work also demonstrated that patients with metastatic EGFR mutant NSCLC had inferior survival when treated with ICI as compared with docetaxel in the second-line setting [Citation25]. Although the majority of these patients were nonsmokers, we are unable to account for the potential confounding effect of EGFR mutation status without access to patient level data. However, it is important to note that only 2% of all patients in our NSCLC cohort had known EGFR mutations and these patients accounted for less than 1% of all patients with advanced cancer included in this meta-analysis. In the HNC and UC cohorts, these patients are not known to have driver mutations that could affect responses to ICI. Therefore, the impact of EGFR mutation in NSCLC on the overall result of this analysis would probably be minimal. We also could not detect a significant difference in ICI effectiveness by sex or age, suggesting that the confounding impact of these factors, if any, would be minimal. Furthermore, given that the included studies were large randomized trials, we expect potential confounders to be equally distributed between the randomized arms and hence impact minimally on estimates of relative treatment effects.
In this meta-analysis where never-smokers are relatively under-represented, our results highlight the importance of stratifying patients by smoking status in all future trials of ICI, especially in the absence of a clear predictive molecular biomarker. Our literature search found no report of smoking history in trials investigating ICI in the treatment of non-tobacco-implicated cancers. A standardized definition of smoking status, such as those defined by the National Health Interview Survey is needed for all ICI oncology trials. This includes better quantification of smoking exposure such as a objectively quantifying smoking history, or using objective tests such as cotinine levels in body fluid [Citation26]. Ongoing research is required to better understand the molecular basis of treatment effect modification by smoking in ICI therapy.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download PDF (1.7 MB)Disclosure statement
The authors report no conflicts of interest.
References
- Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856.
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214.
- Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124–128.
- Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206.
- Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–1134.
- Gaykalova DA, Mambo E, Choudhary A, et al. Novel insight into mutational landscape of head and neck squamous cell carcinoma. PLos One. 2014;9(3):e93102.
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639.
- Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab vs docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(8):1156–1170.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135.
- Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426.
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). JCO. 2017;35(35):3924–3933.
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846.
- Smith DA, Vansteenkiste JF, Fehrenbacher L, et al. Updated survival and biomarker analysis of a randomised phase II study of atezolizumab versus docetaxel in 2L/3L NSCLC (POPLAR). JCO. 2016;34(15_suppl):9028.
- Ferris RL, Blumenschein G, Jr., Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867.
- Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Two year follow-up from the phase 3 KEYNOTE-045 trial of pembrolizumab versus investigator’s choice (paclitaxel, docetaxel, or vinflunine) in recurrent, advanced urothelial cancer. Presented at the American Society of Clinical Oncology-2018 Genitourinary Cancers Symposium; February 8–10, 2018. San Francisco, CA.
- Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757.
- Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab versus platinum-based chemotherapy as first-line therapy for advanced/metastatic NSCLC with a PD-L1 TPS = >1%: open label Keynote-042 study. Presented at American Society of Clinical Oncology Annual Meeting; June 1–5, 2018. Chicago, IL.
- Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced non-small cell lung cancer: results of the phase 3 CheckMate 078 Study. Presented at American Association for Cancer Research Annual Meeting; April 14–18, 2018. Chicago, IL.
- Soulieres D, Cohen E, Le Tourneau C, et al. Updated survival results of the KEYNOTE-040 study of pembrolizumab vs standard-of-care chemotherapy for recurrent or metastatic head or neck squamous cell carcinoma. Presented at American Association for Cancer Research Annual Meeting; April 14–18, 2018. Chicago, IL.
- Lee C, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–216.
- Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2287–2293.
- Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018; 24(9):1441–1448.
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med.. 2018;378(22):2093–2104.
- Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):737–746.
- Elias R, Karantanos T, Sira E, et al. Immunotherapy comes of age: Immune aging and checkpoint inhibitors. J Geriatr Oncol. 2017;8(3):229–235.