Erdheim–Chester disease (ECD) is a rare non-Langerhans histiocytosis with a broad, nonspecific clinical spectrum from asymptomatic to life-threatening multi-organ involvement [Citation1,Citation2]. Diagnosis of ECD is made with histology and phenotype of histiocytes in appropriate clinical and radiological context [Citation3]. Somatic activating mutations of BRAFV600E are detected in 50–70% ECD patients [Citation2,Citation4,Citation5]. The BRAF inhibitor vemurafenib has shown dramatic clinical and radiographic efficacy and changed the initial treatment strategy [Citation6]. However, the estimated annual cost of vemurafenib is approximately 50,000 dollars in China, which largely restricts its availability for most Chinese patients. Historically, IFN-α has been widely used with variable efficacy as the treatment for ECD [Citation7]. The estimated annual cost of IFN-α is approximately 1600 dollars in China, and the relatively low price makes it the first option for ECD patients in low-income countries. ECD with central nervous system (CNS) involvement is related to poor prognosis [Citation8]. We found that IFN-α treatment did not change CNS involvement as a single predictor for poor survival [Citation5]. Therefore, new treatment strategies are urgently needed for ECD patients with CNS involvement.
Cytarabine is an important drug in the treatment of histiocytosis, including adult Langerhans cell histiocytosis [Citation9] and non-LCH like systemic juvenile xanthogranulomatosis [Citation10]. We tried to use intermediate-dose cytarabine as salvage therapy for a wild-type BRAF ECD patient who relapsed in CNS during treatment with IFN-α, and the patient had a remarkable response to this therapy [Citation11]. Since then, we started to explore the possibility of cytarabine as first-line therapy to treat CNS involved ECD. In the current study, we reported 2 ECD patients with severe CNS lesions who achieved favorable therapeutic effects with intermediate-dose cytarabine.
Case 1
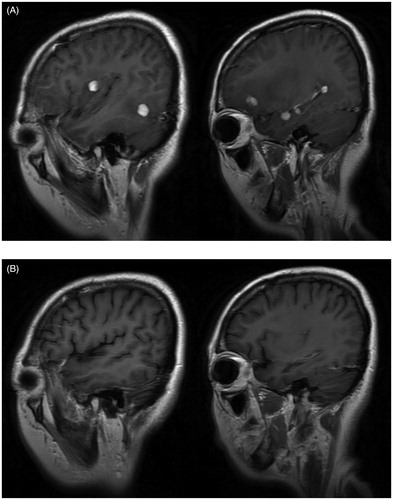
A 44-year-old female presented with headache, polyuria, pseudosphresia for nearly one year. Head magnetic resonance imaging (MRI) showed multiple nodular enhancement lesions in the brain, located in the bilateral frontal lobe, bilateral occipital lobe, bilateral temporal lobe, right insular lobe, right basal ganglia and right parietal lobe, with long T1 and equal T2 signals, surrounded by edema (). She underwent an operation to resect an intracranial lesion in the left frontal lobe. Histological examination of the mass demonstrated infiltration of typical histiocytes consistent with ECD with immunohistochemical staining positive for CD68, S100-dim and negative for CD1a. The BRAFV600E mutation was negative detected by PCR and immunohistochemistry as previously described [Citation2]. A 99mTechnetium methylene di-phosphonate (99 m Tc-MDP) bone scintigraphy scan revealed intense, symmetrical osteoblastic activity along the surfaces of bilateral distal femur and proximal tibia. Based on typical imaging findings and pathological results, a diagnosis of Erdheim–Chester disease was made. After signing the informed consent, she received four courses of cytarabine 500 mg/m2 by 3-hour infusion every 12 hours for 3 days every 5 weeks. The symptoms of headache, pseudosphresia were completely relieved after four-course of this monotherapy. Repeated head MRI suggested those multiple abnormal signal lesions in the brain were smaller and the surrounding edema was narrower (). No major adverse events occurred except for IV°myelosuppression and mild fever, which were managed by Granulocyte Colony-Stimulating Factor (G-CSF), blood transfusion and antibiotics. The patient continues to receive IFN-α 900 MIU every two days as maintenance therapy and her condition remained stable after 23 months of follow-up since diagnosis.
Case 2
A 23-year-old female had a sudden onset of blurred vision just after one week of her accouchement. Ten months later, she developed striking exophthalmos and blindness of her right eye. We gave her steroid pulse therapy and vision of the right eye was transiently recovered, but gradually worsened until loss of light perception. Head Magnetic Resonance Imaging (MRI) showed multiple nodular lesions left frontal lobe, bilateral lateral ventricle triangle and right saddle with long or equal T1 and T2 signals. Fundal examination found congestion and edema of bilateral optic papilla. A left parietal craniotomy was performed for resection of the mass. A right frontal temporal lobe and left frontal lobe tumor resection was performed. Pathology demonstrated large amount of histiocytes with CD68-positive, and CD1a-negative, S-100-negative. BRAFV600E mutation was positive detected by immunohistochemical stain. The 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT revealed highly intense uptake mainly in bilateral lateral ventricle, bilateral cavernous sinus, left temporal lobe and cerebellum (). Contrast CT showed plaques and fibrous stripes in the lungs, abnormal soft tissue density in the lateral margin of the bilateral kidneys indicating ‘hairy kidney’. A 99 mTc-MDP bone scintigraphy scan also suggested the diagnosis of ECD by radiotracer uptake in the distal ends of the femurs, the proximal and distal tibia, humerus and radius.
Figure 2. (A) Initial 18 F–FDG PET/CT scan showing hypermetabolic activity mainly in bilateral lateral ventricle, bilateral cavernous sinus, left temporal lobe and cerebellum. (B) Repeated 18 F-FDG PET/CT showed great decrease of number and size of intracranial lesions with only a high-uptake locus in cave sinus remained.
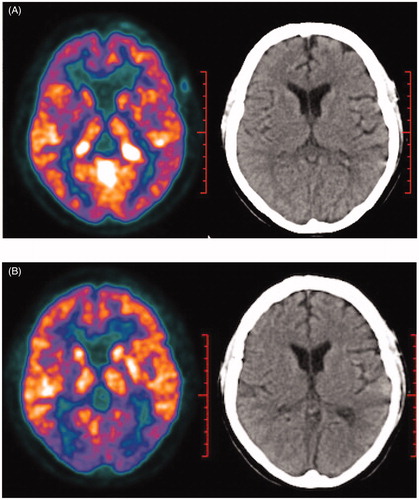
Given the evidence of clinical and radiological presentations as well as histological findings, the diagnosis of CNS-involved ECD was made. After signing the informed consent, the patient received cytarabine 500 mg/m2 by 3-hour infusion every 12 hours for 3 days every 5 weeks. However, she experienced a sudden onset of left eye blindness on the fifth day of first-course cytarabine. Orbit MRI showed massive retrobulbar infiltration which compressed her left optic nerves. Therefore, we tried steroid pulse therapy and gave her IFN-α 900 MIU every two days as combined therapy. After four courses of cytarabine, repeated PET/CT scan showed dramatic improvement of intracranial lesions with only a high-uptake locus in cave sinus remained (). No high-uptake lesion was found in her lung and kidney. However, the patient was still blind with only little light perception. She experienced transient and manageable IV° myelosuppression without severe infection or hemorrhage. IFN-α therapy (900 MIU every 2 days) is continued, and the patient is under close surveillance. So far, she is doing well clinically except blindness in her eyes for more than 11 months after diagnosis.
Discussion
Central nervous system involvement is common in ECD (varying from 25 to 50%) leading to severe functional disability in almost all patients [Citation8,Citation12,Citation13]. Arnaud et al. analyzed a large cohort of 53 ECD patients and revealed that central nervous system involvement was a sole independent clinical predictor of poor survival [Citation8].
During the past two decades, many new therapeutic modalities have been developed. High-dose interferon (IFN)-α/PEG-IFN-α (9 MU × 3 week−1) can significantly improve overall survival compared with other therapies and was an independent predictor of better survival in multivariate analysis [Citation7,Citation8,Citation14]. Interferon-α plays its role by promoting the terminal differentiation of histiocytes and dendritic cells. However, we previously investigated 32 ECD patients who received high dose IFN-α therapy and found that CNS involvement was still a poor prognostic factor with IFN-α monotherapy [Citation5]. This is probably due to poor penetration of IFN-α through the blood–brain barrier, as is known that only ∼0.1% of plasma IFN-a gains access to the CNS [Citation15]. BRAF inhibitor vemurafenib has shown good therapeutic effect in ECD patients with BRAFV600E gain-of-function mutations, which takes up ∼50–70% of all cases [Citation4]. Moreover, the MEK inhibitor Cobimetinib and the IL-1R antagonist were also shown to have efficacy in ECD [Citation16,Citation17]. However, the significance of all these drugs for CNS lesions remains to be elucidated. In addition, the estimated annual cost of vemurafenib is approximately 50,000 dollars in China, which is not covered by health insurance and far beyond what most patients in low-income countries can afford. Therefore, more therapeutic regimens for CNS involved ECD should be further explored.
High-dose cytarabine is an essential drug in the treatment of CNS involvement of acute myeloid leukemia and lymphoblastic leukemia. Significant concentrations of cytarabine are achieved in the cerebrospinal fluid (CSF) during conventional-dose intravenous infusions [Citation18]. LCH and ECD are rare inflammatory myeloid hematopoietic neoplasms which both belong to ‘L’ (Langerhans) group based on clonal mutations involving genes of the MAPK pathway in >80% of cases [Citation3]. Evidence showed that intermediate-dose cytarabine greatly improved the survival of LCH with CNS involvement (One year PFS was 93%) [Citation19]. We previously reported the successful salvage therapy with cytarabine in the treatment of CNS-relapsed ECD without BRAFV600E mutation [Citation11]. Therefore, in the current report, we tried intermediate-dose cytarabine as first-line therapy for CNS involved ECD. As seen in these two cases, patients achieved clinical remission after four courses of cytarabine with or without IFN-α, and the efficacy was confirmed by the relief of symptoms and 18 F-FDG PET/CT or head MRI. Moreover, the two patients, with/without BRAFV600E mutation, achieved good response to the treatment, indicate that cytarabine may be effective to all ECD patients regardless of BRAFV600E mutation. However, our study had some limitations. Both patients received IFN in parallel or as with cytarabine so the therapeutic effect of IFN could not be excluded. Moreover, critical questions, such as is maintenance therapy necessary after intermediate-dose cytarabine treatment, is currently unknown. A well-designed prospective study in the future might give us more information about these questions.
In conclusion, our observation suggests that intermediate-dose cytarabine with or without IFN-α as first-line therapy can be effective for CNS-involved Erdheim–Chester disease, especially for patients without BRAFV600E mutation or cannot afford BRAF inhibitor. More investigations including multicenter perspective studies are warranted to further confirm the efficacy of this traditional but novel therapy.
| Abbreviations | ||
| CNS | = | Central nervous system |
| CT | = | Computed tomography |
| ECD | = | Erdheim–Chester disease |
| LCH | = | Langerhans cell histiocytosis |
| MRI | = | Magnetic resonance imaging |
| PET | = | Positron emission tomography |
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Cavalli G, Guglielmi B, Berti A, et al. The multifaceted clinical presentations and manifestations of Erdheim–Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis. 2013;72(10):1691–1695.
- Cao X, Sun J, Li J, et al. Evaluation of clinicopathologic characteristics and the BRAF V600E mutation in Erdheim-Chester disease among Chinese adults. Ann Hematol. 2016;95(5):745–750.
- Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483–492.
- Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703.
- Cao X-X, Niu N, Sun J, et al. Clinical and positron emission tomography responses to long-term high-dose interferon-α treatment among patients with Erdheim–Chester disease. Orphanet J Rare Dis. 2019;14(1):11.
- Haroche J, Cohen-Aubart F, Emile J-F, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAFV600E-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411–418.
- Braiteh F, Boxrud C, Esmaeli B, et al. Successful treatment of Erdheim-Chester disease, a non-Langerhans-cell histiocytosis, with interferon-alpha. Blood. 2005;106(9):2992–2994.
- Arnaud L, Hervier B, Neel A, et al. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117(10):2778–2782.
- Allen CE, Ladisch S, Mcclain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126(1):26–35.
- Maintz L, Wenzel J, Irnich M, et al. Successful treatment of systemic juvenile xanthogranulomatosis with cytarabine and 2‐chlorodeoxyadenosine: case report and review of the literature. Br J Dermatol. 2017;176(2):481–487.
- Cao X, Niu N, Sun J, et al. Efficacy of intermediate-dose cytarabine in central nervous system-relapsed wild-type BRAF Erdheim-Chester disease. Ann Hematol. 2018;97(1):185–187.
- Haroche J, Arnaud L, Cohen-Aubart F, et al. Erdheim–Chester Disease. Curr Rheumatol Rep. 2014;16(4):412.
- Drier A, Haroche J, Savatovsky J, et al. Cerebral, facial, and orbital involvement in Erdheim-Chester disease: CT and MR imaging findings. Radiology. 2010;255(2):586–594.
- Hervier B, Arnaud L, Charlotte F, et al. Treatment of Erdheim-Chester disease with long-term high-dose interferon-α. Semin Arthritis Rheum. 2012;41(6):907.
- Fioravanti J, Medina-Echeverz J, Ardaiz N, et al. The fusion protein of IFN-α and apolipoprotein A-I crosses the blood–brain barrier by a saturable transport mechanism. J Immunol. 2012;188(8):3988–3992.
- Aubart FC, Emile J, Maksud P, et al. Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br J Haematol. 2018;180(1):150–153.
- Aouba A, Georgin-Lavialle S, Pagnoux C, et al. Rationale and efficacy of interleukin-1 targeting in Erdheim-Chester disease. Blood. 2010;116(20):4070–4076.
- Slevin M, Piall E, Aherne G, et al. The pharmacokinetics of cytosine arabinoside in the plasma and cerebrospinal fluid during conventional and high‐dose therapy. Med Pediatr Oncol. 1982;10(S1):157–168.
- Simko SJ, McClain KL, Allen CE. Up-front therapy for LCH: is it time to test an alternative to vinblastine/prednisone? Br J Haematol. 2015;169(2):299.