Abstract
Introduction: Objective measurements of levels of physical activity and fitness in patients with head and neck cancer (HNC) are lacking. Furthermore, demographic, clinical and lifestyle-related correlates of low levels of physical activity and fitness in patients with HNC are unknown. This study aims to investigate the levels of accelerometer that assessed physical activity and fitness in patients with HNC and to identify their demographical, clinical and lifestyle-related correlates.
Methods: Two hundred and fifty-four patients who were recently diagnosed with HNC and participated in the NETherlands QUality of life and Biomedical cohort studies In head and neck Cancer (NET-QUBIC) study were included. Physical activity (accelerometer), cardiorespiratory fitness (Chester Step Test), hand grip strength (hand dynamometer) and lower body muscle function (30-second chair-stand test) were assessed. Multivariable linear regression analyses with a stepwise forward selection procedure were used.
Results: Patients spent 229 min/d in physical activity of which 18 min/d in moderate-to-vigorous physical activity. The mean predicted VO2max was 27.9 ml/kg/min, the mean hand grip strength was 38.1 kg and the mean number of standings was 14.3. Patients with lower educational level, more comorbidity and higher tumor stage spent significantly less time in physical activity. Older patients, females and patients with a higher tumor stage had significantly lower cardiorespiratory fitness levels. Older patients, females, patients with more comorbidity, patients with normal weight and patients who have never smoked had significantly lower hand grip strength. Older patients, patients with lower educational level, smokers and patients with more comorbidity had a significantly lower function of lower body muscle.
Conclusions: Pre-treatment levels of physical activity, cardiorespiratory fitness and lower body muscle function are low in patients with HNC. Based on this study, exercise programs targeted and tailored to patients with low levels of physical activity and fitness can be developed.
Introduction
Head and neck cancer (HNC) comprises different sites of cancer in the head and neck region and accounts for more than 650,000 cases and 330,000 deaths annually [Citation1]. Smoking, alcohol consumption and infection with the human papillomavirus (HPV) are the most common risk factors for developing HNC [Citation2]. Observational studies showed that higher levels of physical activity following the diagnosis and treatment of cancer and higher levels of physical fitness before the diagnosis of cancer are associated with reduced mortality [Citation2–4] and better quality of life [Citation5], but the relationship is not uniform, may differ by the type of cancer. Based on these studies, it seems clear that levels of physical activity and fitness play an important role in the risk of cancer, the quality of life of patients with cancer and mortality in patients with cancer.
Physical activity is a behavior that includes occupational, leisure, household or other activities, whereas health-related physical fitness is a set of attributes that people have or achieve and which includes cardiorespiratory fitness and muscle strength [Citation6]. Previous retrospective studies showed that 31% of patients with HNC met the current physical activity guideline pre-diagnosis, which decreased to 8.5% after diagnosis [Citation7] and declines further during treatment [Citation5,Citation8,Citation9]. However, all these studies measured physical activity through self-report, which is prone to bias [Citation10] and likely to over- or underreport physical activity levels [Citation11]. Unfortunately, objective measurements of physical activity levels in patients with HNC are lacking and physical fitness levels have only been investigated previously in small groups of patients with HNC participating in pilot exercise intervention studies [Citation12,Citation13]. Therefore, objective measurements of levels of physical activity and fitness in a large group of patients with HNC are warranted.
Identifying physically inactive and unfit patients with HNC before the start of treatment is important to timely refer patients to exercise programs because it may lead to an improvement in physical function [Citation12], fatigue [Citation12,Citation14] and quality of life [Citation12]. To target and tailor these exercise programs to subgroups of patients with low levels of physical activity and fitness, it may help to identify their demographic, clinical and lifestyle-related correlates.
Therefore, we aimed to investigate the levels of accelerometer that assessed physical activity and fitness in a large sample of patients with HNC shortly after diagnosis, and to identify the demographic, clinical and lifestyle-related correlates of physical activity and fitness.
Methods
Study design
The current study has a cross-sectional design in which data from participants of the NETherlands QUality of life and Biomedical cohort studies In head and neck Cancer (NET-QUBIC) study was used [Citation15]. The NET-QUBIC study is a longitudinal observational cohort study in 739 newly diagnosed patients with HNC. The research protocol was reviewed and approved by the Medical Ethics Committee of VU University Medical Center and all Boards of participating medical centers. All patients provided written informed consent prior to participation.
Study population
In the current study, we used baseline data from the first 254 patients who were included between February 2014 and June 2016 from eight HNC centers throughout the Netherlands. The data release of the first 254 patients was pre-planned and in the current cross-sectional study these data was used [Citation15]. Baseline assessments took place shortly after the diagnosis of HNC and before the start of treatment. To be eligible for the NET-QUBIC study, patients needed to be (i) diagnosed with HNC (oral cavity, oropharynx, hypopharynx, larynx, unknown primary; all stages), (ii) before start of treatment and (iii) able to read, speak and write the Dutch language. Patients were excluded if they (i) had malignancies of the salivary glands, nasopharyngeal malignancies, lymphoma, skin malignancies or thyroid cancer or (ii) had psychiatric comorbidities (e.g., schizophrenia, Korsakoff’s syndrome, severe dementia).
Main outcomes
The total NET-QUBIC assessment protocol involved three components: (1) patient reported outcome measures, (2) home visit with interviews and tests (including physical fitness); during this home visit patients were provided with materials to collect data of physical activity (accelerometer) and saliva samples and (3) collection of blood and oral rinse samples. Due to logistic reasons not all components could always be performed (e.g., short time between diagnosis and start of treatment). Also, patients were allowed not to complete all three components, if this was too much burden.
Accelerometer assessed physical activity
Patients were instructed to wear an accelerometer (ActiGraph wGT3X) at the hip for seven consecutive days during all waking hours. The accelerometer measures raw accelerations in three axes and is recognized as a reliable and valid tool to assess physical activity in healthy persons [Citation16]. Vertical accelerations were converted into physical activity using several data reduction steps [Citation17]. A valid wear day was defined as ≥10 hours/day of wearing time and non-wearing time as ≥60 min of consecutive zero counts [Citation17]. To be included in the analyses, the number of valid wear days needed to be at least five, including one weekend day. Time spent in total physical activity was expressed as the mean number of minutes in any intensity of physical activity per day (≥100 counts per minute). Moderate-to-vigorous physical activity (MVPA) was defined as ≥1952 counts per minute [Citation17].
Physical fitness
The Chester Step Test was used to predict the maximum oxygen uptake (predicted VO2max). It has shown to be a valid test for the estimation of aerobic capacity in healthy participants and is suitable for use in the patient’s home environment [Citation18]. Participants were instructed to step up and down a single step (height between 15 and 30 cm, depending on age and physical capacity of the patient) to a metronome beat at 60 steps per minute for 2 min, after which both heart rate and rating of perceived exertion (RPE) ranging from 6 (very light) to 20 (exhaustion) were recorded [Citation19]. Step rate then increased by 20 steps/min every next 2 min where after heart rate and RPE were recorded again. The test followed this incremental pattern until patients either reached: (i) a heart rate of 80% of the predicted maximum (220 – age), (ii) an RPE of 14 or (iii) completed the test, i.e., five stages (last stage: 136 steps per minute). Heart rates after completion of each stage were plotted on a graphical datasheet and a visual line of best-fit was drawn between the measured heart rates. This line was extended until it reached the 80% of the maximum heart rate of that patient, which was calculated by subtracting the patient’s age from 220. The point where the drawn line and the 80% maximum heart rate line crossed each other, determined the matching maximal oxygen uptake value. At least two valid heart rate measurements were needed to estimate the maximal oxygen uptake.
Handgrip strength was assessed with a hand grip dynamometer (JAMAR), which has shown to be a valid assessment of upper extremity strength [Citation20]. Participants were instructed to perform a maximal isometric contraction and to complete two consecutive measurements for each hand. The highest value of the four attempts was used as indicator for hand grip strength.
Lower body muscle function was assessed using the functional 30-second chair-stand test, which has shown to be a reliable and valid indicator of lower body function [Citation21]. Participants were instructed to rise to a full stand and return to the original seated position as quickly as possible. The total number of times that the participants raised to a full stand in 30 seconds was reported.
Demographic factors
Educational level and living status were assessed with an interview or through questionnaires. Any educational level equal or lower than ‘lower or preparatory vocational education’ was defined as a low level of education. Living status was dichotomized into living with someone (e.g., partner, (grand)child) versus living alone.
Clinical factors
Body height and weight were assessed during a home visit at the patient’s home and body mass index (BMI) was calculated (body weight/height2, kg/m2). A BMI between 18.5 and 25 kg/m2 was defined as normal weight, a BMI below 18.5 kg/m2 as underweight, a BMI above 25 kg/m2 as overweight and a BMI above 30 kg/m2 as obesity. Primary tumor site, tumor stage and human papilloma virus (HPV) status were retrieved from medical records. Based on clinical relevance, tumor stage was dichotomized into stage I–III versus stage IV. Comorbidity was assessed with the Adult Comorbidity Evaluation-27 (ACE-27) based on data retrieved from the medical record, resulting in an overall score of none, mild, moderate or severe [Citation22]. Subsequently, this overall comorbidity score was dichotomized into none/mild versus moderate/severe.
Lifestyle-related factors
Smoking habits and alcohol consumption were assessed with a study-specific questionnaire. Patients who had never smoked or drank alcohol on a daily basis were labeled as having no history of smoking or alcohol consumption, respectively. Patients who had previously smoked or drank alcohol but did not smoke or drank alcohol currently were labeled as having a history of smoking or alcohol consumption, respectively. All patients who smoked or consumed alcohol on a daily basis, were labeled as smokers and consumers of alcohol, respectively.
Statistical analyses
Linear regression analyses were conducted to identify variables that were significantly associated with total time spent in physical activity, cardiorespiratory fitness, hand grip strength and lower body muscle function, with separate models for each continuous outcome measure. Prior to the multivariable analyses, we checked whether multicollinearity (r ≥ 0.60) was present between the potential correlates, but this was not the case. Furthermore, assumptions of linear regression analyses were checked and met. A stepwise forward selection procedure was used to build the multivariable regression models, starting with the variable that was most strongly associated with the outcome in the univariable regression model. Subsequently, the next strongest variable was selected after controlling for the first variable. This procedure was repeated until no variables with an association with the outcome at a significance level of p<.10 could be added to the model. We used a significance level of <0.10 to avoid missing important correlates when building the model [Citation23]. The regression coefficients (β) with 95% confidence interval (CI) and corresponding p values of the final models were reported. The regression coefficients reflect the absolute difference between the two categories of a variable. As levels of cardiorespiratory fitness [Citation24] and hand grip strength [Citation25] differ between males and females, we performed a sensitivity analysis studying correlates for these outcomes separately for men and women. To check whether missing values were selective, we performed logistic regression analyses to study differences in demographic, clinical and lifestyle-related characteristics between the patients with missing values and those without. Due to the high number of variables, we only included variables in the multivariable regression model of which the association with missings had a p value <.25 in the univariable model. All analyses were conducted with SPSS version 22 (SPSS Inc., Chicago, IL, USA).
Results
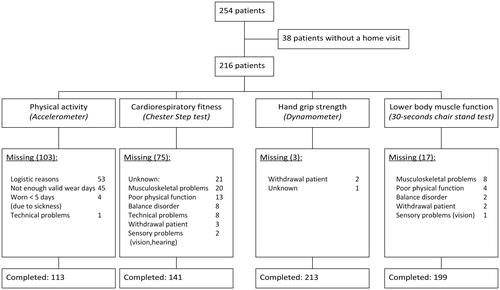
In total, 254 patients were included in the NET-QUBIC study and in 38 patients a home visit was not performed and thus had no measurements of physical activity and fitness. Women were more likely to have no data on home visits [odds ratio (OR)=0.37, 95%CI 0.18 to 0.75, p<.01].
The mean age of the 216 patients that was included in this study was 62 years (SD 9.8) and 75% were men (). The proportion of patients that completed the measurements of physical activity, cardiorespiratory fitness, hand grip strength and lower body muscle function was 52, 65, 99 and 92%, respectively (). Most frequent reasons for incomplete measurements were: insufficient time left for the accelerometer measurements prior to start of treatment (51%) and musculoskeletal impairments (27% and 47% for the Chester Step Test and 30-second chair-stand test, respectively) (). presents differences between patients with and without missing values for physical activity as this outcome had the largest proportion of missing values. There were no variables significantly and independently associated with missing data on physical activity, nor for hand grip strength and lower body muscle function (data not shown). Patients with a valid Chester Step Test were more likely to be younger (OR = 0.92, 95%CI = 0.87 to 0.97, p<.01) and have less comorbidity (OR = 0.31, 95%CI = 0.13 to 0.74, p=.01) than patients without a valid Chester Step Test.
Table 1. Demographic, clinical and lifestyle related characteristics and physical activity and fitness of patients with at least one valid measurement of physical activity or fitness (n = 216).
Table 2. Demographic, clinical and lifestyle related characteristics of participants with accelerometer data and without accelerometer data.
Patients spent on average 229 min/d in physical activity of which on average 18 min/d in MVPA. The mean predicted VO2max was 27.9 ml/kg/min, the mean hand grip strength was 38.1 kg, and the mean number of stands was 14.3 times ().
Multivariable regression analyses showed that patients with a lower educational level, a higher level of comorbidity and a higher tumor stage spent significantly less time in physical activity (). Patients with a higher tumor stage and a higher comorbidity level spent less time in MVPA.
Table 3. Univariable and multivariable regression analyses of potential correlates of accelerometer assessed physical activity and fitness.
Older patients, females and patients with a higher tumor stage had significantly lower cardiorespiratory fitness levels (). Sensitivity analyses stratified for gender did not yield any other correlates.
Older patients, females, patients with more comorbidity, patients with a normal weight (compared to patients with overweight and obesity, patients with no history of smoking (compared to patients with a history of smoking) and patients living alone had significant lower hand grip strength (). Sensitivity analyses stratified for gender did not yield any other correlates.
Older patients, patients with a low educational level, smokers and patients with more comorbidity had a significant lower function of the lower body muscle ().
Discussion
This study investigated the levels and the demographic, clinical and lifestyle-related correlates of accelerometer that assessed physical activity and fitness in a relatively large group of newly diagnosed patients with HNC.
Our finding that newly diagnosed patients with HNC before start of treatment spent on average 229 min/d in physical activity is substantial lower than the 375 min found in healthy persons who were slightly older [Citation26] and the 296–323 min in long-term survivors of various types of cancer in the same age range [Citation27,Citation28]. Also the 18 min/d spent in MVPA, was lower than 26 min reported in one study among cancer survivors with a mean age of 59 years [Citation28], but was comparable to the 16 min found in another study among cancer survivors with a mean age of 61 years [Citation27]. A possible explanation for the lower levels of physical activity and MVPA in this sample might be the recent diagnosis of cancer with companying psychosocial impact. Furthermore, unhealthy lifestyle habits like smoking and alcohol drinking which are specific for this tumor type tend to cluster with physical inactivity [Citation29]. The estimated cardiorespiratory fitness level (mean 27.9 ml/kg/min) of patients in this study was lower than the measured VO2max of 33.7 ml/kg/min reported in healthy populations [Citation30], but higher than the directly measured VO2max of 23.7 ml/kg/min in patients with cancer during or following treatment [Citation31]. However, previous research has shown that submaximal exercise tests, especially in participants with low levels of physical fitness, overestimate the actual measured exercise capacity [Citation32]. Furthermore, patients in this study who completed the Chester Step Test were significantly younger and had less comorbidity than patients who did not complete the test. The mean hand grip strength for women and men in this study was comparable to the grip strength found in healthy elderly [Citation33] and in slightly younger patients with different types of cancer during or after treatment [Citation31]. The 14 times standing during the 30-second chair-stand test, was slightly lower than the 17 times reported in patients during or following treatment for different types of cancer [Citation31], which might be explained by the younger age in the latter study. Overall, results showed that newly diagnosed patients with HNC before treatment have lower levels of physical activity, cardiorespiratory fitness and lower body muscle function compared with the general population and survivors with various types of cancer, but comparable hand grip strength.
The low physical activity and fitness levels before the start of treatment found in this study need further attention, because, in general, these levels are likely to decrease further during treatment [Citation7]. An exercise intervention is currently not part of routine care in patients with HNC, although it may improve physical function, fatigue and quality of life [Citation12,Citation34]. However, more research is needed into the feasibility and effectiveness of exercise interventions targeting HNC patients before, during and after treatment.
The present study provides correlates of low levels of physical activity and fitness. Our finding that patients with a lower educational level were less physically active and had lower function of the lower body muscle has been shown in previous studies among patients with breast and colon cancer [Citation35,Citation36]. The finding that older patients had lower cardiorespiratory fitness levels, lower hand grip strength and reduced lower body muscle function is consistent with previous studies in patients with other cancer types [Citation37,Citation38]. In contrast to previous studies among patients with HNC that used self-reported measures to assess physical activity [Citation39], we found no significant association between age and physical activity. This discrepancy might be due to the fact that low intensity activities, which are typical for elderly, are challenging to estimate correctly by self-report [Citation40], or by the reasonable number of patients that did not complete the accelerometer measurements. These findings indicate that interventions to improve physical activity and fitness should be particularly targeted at and tailored to patients with HNC who are older and have lower educational level, especially because these patients are less reached with existing interventions aiming to improve physical activity and fitness [Citation41]. Female gender was associated with lower levels of cardiorespiratory fitness and lower hand grip strength, which was in line with earlier studies [Citation37,Citation38]. Furthermore, the positive association between BMI and hand grip strength, was also in line with an earlier study [Citation42].
The findings in this study that patients with metastatic cancer had lower levels of accelerometer that assessed physical activity and cardiorespiratory fitness are in line with previous studies, where, for example, breast and kidney cancer survivors with an early disease stage were more likely to meet physical activity guidelines than survivors with an advanced disease stage [Citation43]. Additionally, patients with metastatic breast cancer had significantly lower levels of cardiorespiratory fitness than patients with less advanced stages of disease [Citation44]. Our finding that patients with more comorbidity spent less time in physical activity and had lower levels of hand grip strength and lower body muscle function is in line with the results of previous studies. More comorbidity and a higher tumor stage might be accompanied by a higher symptom burden of these patients, which may be associated with functional impairment and lower levels of physical activity and fitness [Citation45]. Future exercise interventions should be optimally tailored to patients with comorbidities and a higher tumor stage [Citation46].
Surprisingly, a history of smoking was associated with a higher hand grip strength compared to no history of smoking in this study, while a previous study reported negative associations between smoking and hand grip strength [Citation47]. A possible explanation might be that (former) smokers were more involved in manual labor compared with patients who have never smoked, resulting in higher grip strength [Citation48]. Furthermore, smoking was associated with a reduced lower body muscle function in this study, which may be related to reduced skeletal muscle oxidative capacity, blood flow and strength [Citation49]. On the other hand, we found no significant association between smoking behavior and cardiorespiratory fitness [Citation50], which might be due to lower variance among patients who had completed the Chester Step Test.
Strengths of this study are the large sample size of patients with HNC all measured before start of treatment and the use of accelerometers to assess physical activity. The relatively large number of missing values on physical activity and/or fitness measurements is a limitation of this study, which limits generalizability to older patients with more comorbidities. Due to home-based assessments, we used the submaximal step test to estimate cardiorespiratory fitness instead of direct measurements, which may have overestimated levels of physical fitness.
Conclusions
In conclusion, pre-treatment levels of cardiorespiratory fitness, lower body muscle function and time spent in total and MVPA are low in patients with HNC. A higher age, female gender, higher tumor stage, lower educational level and more comorbidity were associated with lower levels of objective measurements of physical activity and fitness in patients with HNC. Based on this study, exercise programs can be particularly targeted and tailored to older, less educated patients with comorbidities and higher tumor stage, because these patients are specifically at risk for inactivity and low fitness levels and often do not participate in an exercise program [Citation41].
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27(12):1969–1975.
- Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815–840.
- Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015;26(2):272–278.
- Sammut L, Fraser LR, Ward MJ, et al. Participation in sport and physical activity in head and neck cancer survivors: associations with quality of life. Clin Otolaryngol. 2016;41(3):241–248.
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131.
- Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer. 2006;14(10):1012–1019.
- Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity correlates and barriers in head and neck cancer patients. Support Care Cancer. 2008;16(1):19–27.
- Lønbro S, Dalgas U, Primdahl H, et al. Lean body mass and muscle function in head and neck cancer patients and healthy individuals – results from the DAHANCA 25 study. Acta Oncol. 2013;52(7):1543–1551.
- Schmier JK, Halpern MT. Patient recall and recall bias of health state and health status. Expert Rev Pharmacoecon Outcomes Res. 2004;4(2):159–163.
- Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(Suppl. 2):1–14.
- Rogers LQ, Anton PM, Fogleman A, et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck. 2013;35(8):1178–1188.
- Lønbro S, Dalgas U, Primdahl H, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy – results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108(2):314–319.
- Aghili M, Farhan F, Rade M. A pilot study of the effects of programmed aerobic exercise on the severity of fatigue in cancer patients during external radiotherapy. Eur J Oncol Nurs. 2007;11(2):179–182.
- Verdonck-de Leeuw IM, Jansen F, Brakenhoff RH, et al. Advancing interdisciplinary research in head and neck cancer through a multicenter longitudinal prospective cohort study: the NETherlands QUality of life and BIomedical Cohort (NET-QUBIC) data warehouse and biobank. BMC Cancer. 2019;19(1):765.
- Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity (Silver Spring). 2007;15(10):2371–2379.
- Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47(9):1821–1845.
- Bennett H, Parfitt G, Davison K, et al. Validity of submaximal step tests to estimate maximal oxygen uptake in healthy adults. Sports Med. 2016;46(5):737–750.
- Shephard RJ, Bouchard C. A new approach to the interpretation of Canadian Home Fitness Test scores. Can J Appl Physiol. 1993;18(3):304–316.
- Bohannon RW. Hand-grip dynamometry provides a valid indication of upper extremity strength impairment in home care patients. J Hand Ther. 1998;11(4):258–260.
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119.
- Rogers SN, Aziz A, Lowe D, et al. Feasibility study of the retrospective use of the Adult Comorbidity Evaluation index (ACE-27) in patients with cancer of the head and neck who had radiotherapy. Br J Oral Maxillofac Surg. 2006;44(4):283–288.
- Twisk JWR. Inleiding in de Toegepaste Biostatistiek. Houten: Bohn Stafleu en van Loghum; 2017.
- Wang CY, Haskell WL, Farrell SW, et al. Cardiorespiratory fitness levels among US adults 20–49 years of age: findings from the 1999–2004 National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;171(4):426–435.
- Ahrenfeldt LJ, Scheel-Hincke LL, Kjaergaard S, et al. Gender differences in cognitive function and grip strength: a cross-national comparison of four European regions. Eur J Public Health. 2018;29(4):667–674.
- Dohrn IM, Sjöström M, Kwak L, et al. Accelerometer-measured sedentary time and physical activity-A 15 year follow-up of mortality in a Swedish population-based cohort. J Sci Med Sport. 2018;21(7):702–707.
- Thraen-Borowski KM, Gennuso KP, Cadmus-Bertram L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLoS One. 2017;12(8):e0182554.
- Sweegers MG, Boyle T, Vallance JK, et al. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int J Behav Nutr Phys Act. 2019;16(1):66.
- Schuit AJ, van Loon AJ, Tijhuis M, et al. Clustering of lifestyle risk factors in a general adult population. Prev Med. 2002;35(3):219–224.
- Myers J, Kaminsky LA, Lima R, et al. A reference equation for normal standards for VO2 Max: analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog Cardiovasc Dis. 2017;60(1):21–29.
- Sweegers MG, Altenburg TM, Brug J, et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: a meta-analysis of individual patient data. Br J Sports Med. 2018;53(13):812.
- Stuiver MM, Kampshoff CS, Persoon S, et al. Validation and refinement of prediction models to estimate exercise capacity in cancer survivors using the steep ramp test. Arch Phys Med Rehabil. 2017;98(11):2167–2173.
- Desrosiers J, Bravo G, Hebert R, et al. Normative data for grip strength of elderly men and women. Am J Occup Ther. 1995;49(7):637–644.
- Samuel SR, Maiya GA, Babu AS, et al. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res. 2013;137(3):515–520.
- Lynch BM, Boyle T, Winkler E, et al. Patterns and correlates of accelerometer-assessed physical activity and sedentary time among colon cancer survivors. Cancer Causes Control. 2016;27(1):59–68.
- Forbes CC, Blanchard CM, Mummery WK, et al. A comparison of physical activity correlates across breast, prostate and colorectal cancer survivors in Nova Scotia, Canada. Support Care Cancer. 2014;22(4):891–903.
- Persoon S, Kersten MJ, Buffart LM, et al. Health-related physical fitness in patients with multiple myeloma or lymphoma recently treated with autologous stem cell transplantation. J Sci Med Sport. 2017;20(2):116–122.
- Norman K, Stobaus N, Smoliner C, et al. Determinants of hand grip strength, knee extension strength and functional status in cancer patients. Clin Nutr. 2010;29(5):586–591.
- Buffart LM, de Bree R, Altena M, et al. Demographic, clinical, lifestyle-related, and social-cognitive correlates of physical activity in head and neck cancer survivors. Support Care Cancer. 2018;26(5):1447–1456.
- Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37(3):197–206.
- van Waart H, van Harten WH, Buffart LM, et al. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology. 2016;25(8):964–970.
- Kilgour RD, Vigano A, Trutschnigg B, et al. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer. 2013;21(12):3261–3270.
- Trinh L, Larsen K, Faulkner GE, et al. Social–ecological correlates of physical activity in kidney cancer survivors. J Cancer Surv. 2016;10(1):164–175.
- Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–2537.
- Pandya C, Magnuson A, Flannery M, et al. Association between symptom burden and physical function in older patients with cancer. J Am Geriatr Soc. 2019;67(5):998–1004.
- van der Leeden M, Huijsmans RJ, Geleijn E, et al. Tailoring exercise interventions to comorbidities and treatment-induced adverse effects in patients with early stage breast cancer undergoing chemotherapy: a framework to support clinical decisions. Disabil Rehabil. 2018;40(4):486–496.
- Al-Obaidi S, Al-Sayegh N, Nadar M. Smoking impact on grip strength and fatigue resistance: implications for exercise and hand therapy practice. J Phys Act Health. 2014;11(5):1025–1031.
- Tammelin T, Näyhä S, Rintamäki H, et al. Occupational physical activity is related to physical fitness in young workers. Med Sci Sports Exerc. 2002;34(1):158–165.
- Orlander J, Kiessling KH, Larsson L. Skeletal muscle metabolism, morphology and function in sedentary smokers and nonsmokers. Acta Physiol Scand. 1979;107(1):39–46.
- de Borba AT, Jost RT, Gass R, et al. The influence of active and passive smoking on the cardiorespiratory fitness of adults. Multidiscip Respir Med. 2014;9(1):34.