Abstract
Background: Survival in sarcoma patients depends on a range of prognostic factors. An association between cancer survival and socioeconomic position is known for several other cancers. We therefore examined the relations between three socioeconomic factors and risk of presenting with known tumour related prognostic factors, and the overall mortality of the different socioeconomic and prognostic factors in 1919 patients diagnosed with sarcoma in Denmark 2000–2013.
Material and methods: Patients with sarcoma in extremities or trunk wall aged 30 years or more at diagnosis were identified in the Danish Sarcoma Registry and linked on an individual level to Danish national registries. We obtained data on educational level, disposable income and cohabitation status. Odds ratios (ORs) were estimated for the association between the socioeconomic factors and grade, stage and tumour size. Hazard ratios (HRs) were estimated using Cox proportional hazard models.
Results: In adjusted analyses, educational level, income and cohabitation status were not associated with high grade or dissiminated stage at time of diagnosis. However, living alone was statistically significantly associated with having a large soft tissue sarcoma (≥5 cm) at time of diagnosis (OR 1.51; CI1.12–2.03). The overall mortality was statistically significantly increased in the group of patients with ≤10 years of education (HR 1.27; CI 1.02–1.57), in patients with the 20% lowest income (HR 1.30; CI 1.00–1.67) and nearly in patients living alone (HR 1.16; CI 0.99–1.36).
Conclusion: In this nationwide, multicentre, population-based study, soft tissue sarcoma patients living alone had greater risk of having a large tumour at time of diagnosis. Soft tissue and bone sarcoma patients with a short education, low income, or living alone, had a higher mortality. This might indicate that the social differences in mortality might be related to treatment aspects and the biology of the disease rather that the diagnostic process.
Introduction
Sarcomas are a rare, heterogeneous group of malignancies that represents 1% of all newly diagnosed cancers [Citation1,Citation2]. Except for rare genetic defects and ionising radiation, the aetiology of sarcomas remains generally unknown [Citation2]. Grade at the time of diagnosis is one of the most important prognostic factors [Citation3–6]. Several important prognostic factors have been established: large tumour size, disseminated stage, high grade, and presence of comorbidity [Citation5,Citation7,Citation8]. With no clear aetiology, it is important to investigate and identify possible non-biological prognostic factors in order to improve early detection, treatment and outcome [Citation2].
Social and economic factors influence the position an individual holds within a society [Citation9,Citation10]. General symptom awareness, pattern of healthcare seeking, communication with healthcare professionals, compliance to treatment or rehabilitation, can all be influenced by socioeconomic position (SEP). This might result in delayed diagnosis and poorer outcome in patients with low SEP [Citation9,Citation10]. Lifestyle and risk factors such as smoking, physical activity, occupational exposure, dietary habits, and alcohol consumption may be part of the explanation in socioeconomic differences in cancer incidence and survival, but results have been inconsistent [Citation11–14].
In Denmark, the healthcare system is tax-funded and provides universal and free access to general practice as well as in- and outpatient hospital care, although physiotherapy, dentistry, and prescribed medicine have a degree of co-payment [Citation15]. With extensive welfare and universal access to healthcare services, it is expected that patients should achieve the same outcome, regardless of SEP. However, there is a negative influence by SEP on cancer survival in cervix, head and neck, lung and gastric cancers [Citation16–19]. On the contrary the risk of cancer is lower in cancers such as breast, prostate, colon and malignant melanoma [Citation16]. In general, patients with lower SEP have consistently poorer survival than that of higher SEP [Citation16]. Moreover, this socioeconomic gradient appears to be more outspoken in Denmark, compared to other northern European countries [Citation20,Citation21].
A few studies have found that SEP is strongly related to risk of sarcoma [Citation22–24]. Few studies have found that socioeconomic deprivation affects survival of sarcoma patients negatively [Citation23,Citation25–28]. In order to improve the outcome in sarcoma patients, the aim of this nationwide multicentre study was to examine the association between socioeconomic factors (educational level, disposable income and cohabitation status) and three prognostic factors (malignancy grade, stage, and size) in Danish sarcoma patients from 2000–2013, and further to examine the overall mortality in relation to SEP.
Material and methods
Study population and registry linkage
A population-based cohort study was performed based on nationwide Danish registries.
Treatment of sarcomas in Denmark is centralised and standardised. The Danish Sarcoma Registry (DSR) is a validated, population-based, national clinical database administered by the Danish Sarcoma Group, supported by Danish Regions [Citation29]. DSR contains all patients diagnosed with a sarcoma in the extremities or trunk wall from 2000 and onwards [Citation29,Citation30]. DSR comprises patient characteristics and detailed data on tumour characteristics, treatment, follow-up, local and distant recurrences as well as death [Citation29,Citation30]. The database is validated continuously in accordance with applicable rules of the Danish Clinical Registries, RKKP.
In the DSR, we identified 1919 patients with soft tissue or bone sarcoma in the extremities or trunk wall diagnosed between 2000 and 2013, aged 30 years or more at the date of diagnosis and living in Denmark one year before diagnosis. Age was divided into three groups: 30–49 years, 50–69 years and ≥70 years. Patients below the age of 30 were excluded, as they may not have reached their final educational level and thus achieved their final SEP.
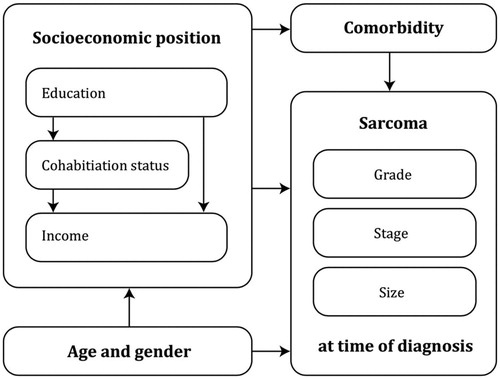
From the DSR, we obtained data on grade, stage, and tumour size at time of diagnosis. Hypothesised mediators and confounders were, based on a literature search, drawn into a causal diagram (). Low-grade sarcoma was defined as histological grade I, and high-grade as histological grade II + III according to the NCI and FNCLCC histopathological grading systems [Citation31]. Based on the WHO Classification of Soft Tissue Tumours and TNM Classification, the cut-off values regarding size were defined as a large soft tissue sarcoma if ≥ 5 cm, and a large bone sarcoma if ≥ 8 cm [Citation8,Citation31]. Disseminated stage at the time of diagnosis was defined as any metastases, both local and distant. Comorbidity was defined as the presence of comorbidity according to the 19 different medical conditions in the Charlson Comorbidity Index (CCI) obtained from the National Patient Registry. The register contains information on all hospital admissions and discharges, and reporting is mandatory by law. Certain discharge diagnoses in the National Patient Registry including the diagnosis in the CCI has been validated [Citation7,Citation32,Citation33].
Figure 1. Hypothesised causal relations between the socioeconomic position (educational level, disposable income and cohabitation status) and grade, stage and tumour size at time of diagnosis.
The Civil Registration System includes all residents in Denmark and contains information about vital status updated on a daily basis [Citation34]. Every individual has a unique personal identity number (CPR-number) that allows linkage between national registries and clinical databases [Citation34].
Socioeconomic position, SEP
Statistics Denmark manages population-based registries as well as data on different social and economic variables: education, income and cohabitation status [Citation35,Citation36].
Education was categorised according to UNESCO’s International Standard Classification of Education and divided into three groups: low: ≤10 years, middle: >10 and ≤15 years and high educational level: >15 years [Citation35]. Equivalence-weighted income was recorded on the basis of the OECD-modified scale [Citation37]. Disposable income was defined as net household income, adjusted for the number of persons in the household. Income was categorised into three groups: the 20% with the lowest income, the 60% with medium income and the 20% with the highest income. Cohabitation was categorised into two groups: married/cohabiting and living alone [Citation34].
Statistical analyses
Odds ratios (ORs) were estimated in logistic regression models to examine the association between the three socioeconomic factors (educational level, disposable income, and cohabitation status) and age, gender, and comorbidity, and the likelihood of receiving a diagnosis of grade II + III sarcoma against grade I, disseminated disease against localised disease, and a large tumour against a small tumour at the time of diagnosis. Analyses were adjusted for age, gender, education, cohabitation status, income, and comorbidity. Crude and adjusted hazard ratios (HRs) were estimated using Cox proportional hazard models. The proportional hazard assumptions were tested using log-minus-log plots. All tests were two-sided. A p-value below 0.05 was considered statistically significant. Estimates were made with corresponding 95% confidence intervals (CI). All analyses were performed in STATA 14.2 software.
Ethics
The Danish Data Protection Agency (j.nr: 1-16-02-245-14), Statens Serum Institute (FSEID-1729), Statistics Denmark (p.nr: 706699), and the Danish Clinical Registries (j.nr: DSD-2017-03-02) approved this study.
Results
The cohort consisted of 1919 patients diagnosed with sarcoma in Denmark from 2000–2013. Of these, 1058 (55%) patients were men, 1497 (78%) patients had a soft tissue sarcoma, 1695 (88%) patients had localised disease at time of diagnosis, and 1289 (74%) had a high-grade sarcoma. According to CCI, 414 patients (22%) had comorbidity in a 10-year period prior to the diagnosis ().
Table 1. Patient characteristics for the 1919 sarcoma patients diagnosed with sarcoma in Denmark 2000–2013.
Association between SEP and grade, stage and size of the sarcoma at time of diagnosis
A total of 1289 (67%) had a high-grade sarcoma at time of diagnosis. No statistically significant associations were found between SEP and having a high-grade sarcoma in the adjusted analysis, though we observed a tendency of increased likelihood of high grade in soft tissue sarcoma patients with shorter education (OR 1.21; CI 0.87–1.66) ().
Table 2a. Associations (odds ratios (ORs) with corresponding 95% confidence intervals (CI)) between socioeconomic position and having high grade (grade II + III) sarcoma in 1751 patients aged ≥30 years in Denmark, 2000–2013.
Metastatic disease was present in 224 (12%) of the patients at the time of diagnosis. No statistically significant associations were found in either the crude or the adjusted analysis.
A tendency of increased likelihood of advanced stage was seen in soft tissue sarcoma patients with shorter education (OR: 1.20; CI 0.73–1.95) and living alone (OR: 1.24; CI 0.86–1.79) and for bone sarcoma patients with lowest disposable income (OR: 1.43; CI 0.48–4.28) ().
Table 2b. Association (odds ratios (ORs) with corresponding 95% confidence intervals (CI)) between socioeconomic position, and disseminated sarcoma in 1919 patients aged ≥30 years in Denmark, 2000–2013.
A total of 1098 (68%) patients had a large sarcoma (soft tissue sarcoma > 5 cm, and bone sarcoma > 8 cm) at time of diagnosis. Patients living alone had statistically significant higher risk of presenting with a large sarcoma (OR 1.30; CI 1.01–1.67), which was also the case for soft tissue sarcomas (OR 1.51; CI 1.12–2.03). Though not statistically significant, patients with lowest income had an increased OR of 2.43 (CI 0.99–5.95) ().
Table 3. Associations (odds ratios (ORs) with 95% confidence intervals (CI)) between socioeconomic position, and having a larger sarcoma (≥5 and ≥8 cm) at time of diagnosis in 1626 patients aged ≥30 years in Denmark, 2000–2013.
SEP and mortality
For all the socioeconomic variables, there was a statistically significant increased 5-year mortality rate (MR) in lower SEP compared to higher SEP. The overall mortality was statistically significantly increased in the group of patients with ≤10 years of education (HR 1.27; CI 1.02–1.57), in patients with the 20% lowest income (HR 1.30; CI 1.00–1.67) and nearly in patients living alone (HR 1.16; CI 0.99–1.36) ().
Table 4. Mortality rates and crude and adjusted hazard ratios with 95% confidence intervals (CI) for all sarcoma patients (n = 1919) and stratified for socioeconomic position, age, gender, comorbidity, grade, stage, and tumour size in Denmark 2000–2019.
Discussion
Main findings
In this nationwide, population-based study, educational level, income, and cohabitation status, were not associated with high grade or disseminated stage at time of diagnosis. Though, living alone was statistically significantly associated with having a large soft tissue sarcoma at time of diagnosis. The overall mortality was statistically significantly increased by up to 30% in patients with low SEP. Thus, the social disparity in mortality seems to be associated with other aspects than the known biological prognostic factors.
Methodological considerations
Characterising the biological and non-biological risk factors for sarcomas remains a challenge due to the rarity of the disease. The main strength of our study includes the population-based study design, the use of reliable population-based national registries, large sample sizes, and complete follow-up on all patients. Furthermore, the possibility to link data on an individual level to clinical databases and registries, and the equal and uniform health care services provided in Denmark. Potential information bias from the Danish registries the Civil Registration System and Statistics Denmark are considered low.
All original data was collected independently of this study, which decrease the risk of differential misclassification.
We used the highest obtained educational level of the individual patients, which reflects the SEP. However, as the registration started in 1966, some elderly patients may have only a lower educational level registered. The age in the study population varies from 30 to 96 years of age. In the last 50 years there have been a shift in opportunities of educational possibilities: more people get a higher education, and people are sooner educated [Citation38]. Living standards have increased significantly and the degree of urbanisation has increased rapidly. This may leave women, certain minorities, and the more elderly part of the cohort over-represented in the group classified as a lower educational level and disposable income, even when adjusting for age and gender, which could distort the results. No information of the quality of the educational experience, such as analytical abilities and cognitive skills were present in the data. We did not take into account that income typically follows a trajectory curve with age, which leaves the income among younger patients and the oldest patients less reliable. The data on cohabitation status is specific to the index date, and the temporal context leaves it difficult to compare for a wide age span.
Comorbidity was calculated from the CCI, which is widely used and has been validated several times for mortality purposes in cancer cohort studies [Citation39–42]. We based the CCI calculation solely on hospital-based diagnoses extracted from the National Patient Registry. Some of the milder conditions in the CCI, e.g., diabetes without end organ damage, are mainly discovered and treated in general practice, and thus underestimating the level of comorbidity is potential. The severity of some of the conditions included in the CCI may be overestimated, as there have been advances of detecting certain diseases and improvement of treatment, e.g., ulcer disease and AIDS. Presence of comorbidity might affect the treatment strategy in terms of lesser aggressive treatment i.e., if the perioperative mortality risk is considered too high. Certain comorbid patients might not tolerate chemotherapy because of their disease. Other drugs related to the comorbid condition might interact with the chemotherapeutic drugs.
The analysis in this study were made from a general effect model and not to investigate the direct effect of education on for example the tumour size, in which case adjustment should only have been made for known confounders.
Comparison with other studies
Living alone was associated with higher odds of presenting with a large sarcoma among soft tissue sarcoma patients. In other cancers, living alone has been associated with more advanced stage of cancer [Citation17,Citation43,Citation44]. This might have to do with living with a partner might reduce delay of healthcare seeking after onset of symptoms, as they are more prone to discuss symptoms, assistance in navigating in the healthcare system and ensure compliance with treatment [Citation9].
No clear association between SEP and presenting with a high-grade tumour, disseminated disease or a large tumour at time of diagnosis was found in the overall sarcoma population. According to a large study from United States, of 2849 osteosarcoma patient diagnosed with a high-grade sarcoma, the patients living in low-socioeconomic counties presented with metastatic disease and a large tumour (>10cm) more frequently [Citation26]. The substantial healthcare disparities based on socioeconomic position in the American society have previously shown to be more pronounced in both sarcomas and other cancers [Citation28,Citation45,Citation46].
To our knowledge only one study in synovial sarcoma patients has studied the risk of being diagnosed with distant metastases using age, gender, site and income [Citation25]. Income was not associated with an increased risk of metastases at the time of diagnosis, which is in concordance with our findings.
Though no significant differences were found in educational level and the relation to grade, stage and tumour size at time of diagnosis, it is well established that health behaviour is related to educational level [Citation9]. Less knowledge about symptoms and general health as well as a tendency to ignore early symptoms has been observed among patients with shorter education [Citation9]. Disposable income is correlated to education. Educational attainment is typically static when people reach a certain age, whereas income is likely to change throughout life, and therefore, it might be more reliable as a measure of a patients’ current socioeconomic position [Citation9].
Previous studies have shown that 19–25% of Danish sarcoma patients have comorbidity at the time of diagnosis [Citation7,Citation47,Citation48]. Comorbidity may mediate the association between SEP and prognosis, as the prevalence of comorbidity generally is higher among people with lower SEP [Citation10].
We found an increased mortality by up to 30% among the patients with low educational level, low disposable income and living alone at time of diagnosis. This is in concordance with previous studies in sarcomas, where it is shown that socioeconomic deprivation negatively affects the outcome [Citation23,Citation25–27]. Though, most populations in these studies are single-centre cohorts, and studies on specific sarcoma subtypes. Furthermore, the definition of the socioeconomic variables varies extensively in the studies. In other cancer types such as cervical, gastric, head and neck, and lung cancer, there is a negative influence by socioeconomic position on cancer survival [Citation16–19]. This could be due to a less extend of self-care among patients with lower socioeconomic position, or poor communication with healthcare professionals, reduced compliance with treatment, and preventive advice.
More attention to socioeconomic position among sarcoma patients could potentially contribute to level out the social and economic differences in health prognoses. Together with comorbidity, socioeconomic position should be a contributing factor in decision-making and planning of treatment in order to ensure a better prognosis in already vulnerable groups of patients.
Conclusion
In this nationwide, multicentre, population-based study we found that soft tissue sarcoma patients living alone had a statistically significantly greater risk of presenting with a large tumour at time of diagnosis. Sarcoma patients with a short education, low income, who lived alone, had a statistically significant higher mortality. This might indicate that the social differences in mortality might be related to treatment aspects and the biology of the disease rather that the diagnostic process. More attention on socioeconomic factors in the treatment of sarcomas is needed in order to improve survival in patients with lower SEP at time of diagnosis.
Acknowledgments
The authors of this article would like to thank statistician Anders Helles Carlsen for assistance with the statistical analyses and data manager Kaare Rud Flarup for assistance with data management and data retrieval.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Fletcher CDM, Bridge JA, Hogendoorn P, et al. WHO Classification of Tumours of soft tissue and bone. IARC WHO Classification of Tumours, Volume 5. Fourth edition ed. Cambridge, MA: WHO Press; 2013.
- Hui JY. Epidemiology and etiology of sarcomas. Surg Clin North Am. 2016;96(5):901–914.
- Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91(10):1914–1926.
- Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130(10):1448–1453.2.0.CO;2] [https://doi.org/17090186]
- Brennan MF. Lessons learned from the study of soft tissue sarcoma. Int J Surg (London, England). 2013;11(Suppl 1):S8–S10.
- Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, et al. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: a cohort study of 922 consecutive patients. Acta Orthop. 2014;85(3):323–332.
- Raedkjaer M, Maretty-Kongstad K, Baad-Hansen T, et al. The impact of comorbidity on mortality in Danish sarcoma patients from 2000-2013: a nationwide population-based multicentre study. PloS One. 2018;13(6):e0198933.
- Grimer RJ. Size matters for sarcomas! Ann R Coll Surg Engl. 2006;88(6):519–524.
- Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 1). J Epidemiol Community Health. 2006;60(1):7–12.
- Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 2). J Epidemiol Community Health. 2006;60(2):95–101.
- Giskes K, Kunst AE, Benach J, et al. Trends in smoking behaviour between 1985 and 2000 in nine European countries by education. J Epidemiol Community Health. 2005; May59(5):395–401.
- Braaten T, Weiderpass E, Kumle M, et al. Explaining the socioeconomic variation in cancer risk in the Norwegian Women and Cancer Study. Cancer Epidemiol Biomarkers Prev. 2005;14(11):2591–2597.
- Groth MV, Fagt S, Brondsted L. Social determinants of dietary habits in Denmark. Eur J Clin Nutr. 2001;55(11):959–966.
- Engholm G, Palmgren F, Lynge E. [Lung cancer, tobacco smoking and environmental factors in Denmark]. Ugeskr Laeger. 1998;160(5):626–631.
- Gundgaard J. Income-related inequality in utilization of health services in Denmark: evidence from Funen County. Scand J Public Health. 2006;34(5):462–471.
- Dalton SO, Schuz J, Engholm G, et al. Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994-2003: summary of findings. Eur J Cancer (Oxford, England: 1990). 2008;44(14):2074–2085.
- Olsen MH, Bøje CR, Kjaer TK, et al. Socioeconomic position and stage at diagnosis of head and neck cancer - A nationwide study from DAHANCA. Acta Oncol (Stockholm, Sweden). 2015;54(5):759–766.
- Faggiano F, Zanetti R, Costa G. Cancer risk and social inequalities in Italy. J Epidemiol Community Health. 1994;48(5):447–452.
- Nagel G, Linseisen J, Boshuizen HC, et al. Socioeconomic position and the risk of gastric and oesophageal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int J Epidemiol. 2007;36(1):66–76.
- Coleman MP, Gatta G, Verdecchia A, et al. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol. 2003;14(90005):v128–149.
- Sant M, Aareleid T, Berrino F, et al. EUROCARE-3: survival of cancer patients diagnosed 1990-94–results and commentary. Ann Oncol. 2003;14(90005):61v– 118.
- Serraino D, Franceschi S, Talamini R, et al. Non-occupational risk factors for adult soft-tissue sarcoma in northern Italy. Cancer Cause Control. 1991;2(3):157–164.
- Ciccone G, Magnani C, Delsedime L, et al. Socioeconomic status and survival from soft-tissue sarcomas: a population-based study in northern Italy. Am J Public Health. 1991;81(6):747–749.
- Hampras SS, Moysich KB, Marimuthu SP, et al. Socioeconomic factors and the risk for sarcoma. Eur J Cancer Prevent. 2014;23(6):560–565.
- Brennan B, Stiller C, Grimer R, et al. Outcome and the effect of age and socioeconomic status in 1318 patients with synovial sarcoma in the English National Cancer Registry: 1985-2009. Clin Sarcoma Res. 2016;6(1):18.
- Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015;39(4):593–599.
- Miller BJ, Gao Y, Duchman KR. Socioeconomic measures influence survival in osteosarcoma: an analysis of the National Cancer Data Base. Cancer Epidemiol. 2017;49:112–117.
- Lee J, Hoang BH, Ziogas A, et al. Analysis of prognostic factors in Ewing sarcoma using a population-based cancer registry. Cancer. 2010;116(8):1964–1973.
- Jorgensen PH, Lausten GS, Pedersen AB. The Danish sarcoma database. Clin Epidemiol. 2016;8:685–690.
- Maretty-Nielsen K, Aggerholm-Pedersen N, Keller J, et al. Population-based Aarhus Sarcoma Registry: validity, completeness of registration, and incidence of bone and soft tissue sarcomas in Western Denmark. Clin Epidemiol. 2013;5:45–56.
- Neuville A, Chibon F, Coindre JM. Grading of soft tissue sarcomas: from histological to molecular assessment. Pathology. 2014;46(2):113–120.
- Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chron Dis. 1970;23(7):455–468.
- Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11(1):83.
- Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7_suppl):22–25.
- Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7_suppl):91–94.
- Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7_suppl):103–105.
- OECD. Notes on OECD Equivalent Scales [Internet]. OECD; 2011 [cited 2019 Jun 5]. Available from: https://www.oecd.org/els/soc/OECD-Note-EquivalenceScales.pdf
- Denmark S. Population by status of education [Internet]. Statistics Denmark. 2018 [cited 2019 Jun 5]. Available from: https://www.dst.dk/en/Statistik/emner/uddannelse-og-viden/befolkningens-uddannelsesstatus
- Iversen LH, Norgaard M, Jacobsen J, et al. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006 – A population-based cohort study. Dis Colon Rectum. 2009;52(1):71–78.
- Boje CR, Dalton SO, Gronborg TK, et al. The impact of comorbidity on outcome in 12,623 Danish head and neck cancer patients: a population based study from the DAHANCA database. Acta Oncol (Stockholm, Sweden). 2013;52(2):285–293.
- Lund L, Jacobsen J, Norgaard M, et al. The prognostic impact of comorbidities on renal cancer, 1995 to 2006: a Danish population based study. J Urol. 2009;182(1):35–40.
- Nakamura T, Grimer R, Gaston C, et al. The value of C-reactive protein and comorbidity in predicting survival of patients with high grade soft tissue sarcoma. Eur J Cancer (Oxford, England: 1990). 2013;49(2):377–385.
- Groome PA, Rohland SL, Hall SF, et al. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011;47(7):642–647.
- Ibfelt E, Kjaer SK, Johansen C, et al. Socioeconomic position and stage of cervical cancer in Danish women diagnosed 2005 to 2009. Cancer Epidemiol Biomarkers Prev. 2012;21(5):835–842.
- Sun M, Abdollah F, Liberman D, et al. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: a survival analysis. Cancer. 2011;117(18):4277–4285.
- Downing S, Ahuja N, Oyetunji TA, et al. Disparity in limb-salvage surgery among sarcoma patients. Am J Surg. 2010;199(4):549–553.
- Aggerholm-Pedersen N, Maretty-Nielsen K, Keller J, et al. Comorbidity in adult bone sarcoma patients: a population-based cohort study. Sarcoma. 2014;2014:1.
- Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, et al. Prevalence and prognostic impact of comorbidity in soft tissue sarcoma: a population-based cohort study. Acta Oncol (Stockholm, Sweden). 2014;53(9):1188–1196.
