Abstract
Introduction: An increasing number of patients is diagnosed with spinal metastases due to elevated cancer incidence and improved overall survival. Patients with symptomatic spinal bone metastases often receive radiotherapy with or without surgical stabilisation. Patients with a life expectancy of less than 3 months are generally deemed unfit for surgery, therefore adequate pre-treatment assessment of life expectancy is necessary. The aim of this study was to assess new factors associated with overall survival for this category of patients.
Patients and methods: Patients who received radiotherapy for thoracic or lumbar spinal metastases from June 2013 to December 2016 were included in this study. The pre-treatment planning CT for radiotherapy treatment was used to assess the patient’s visceral fat area, subcutaneous fat area, total muscle area and skeletal muscle density on a single transverse slice at the L3 level. The total muscle area was used to assess sarcopenia. Furthermore, data were collected on age, sex, primary tumour, Karnofsky performance score, medical history, number of bone metastases, non-bone metastases and neurological symptoms. Univariable and multivariable cox regressions were performed to determine the association between our variables of interest and the survival at 90 and 365 days.
Results: A total of 310 patients was included. The median age was 67 years. Overall survival rates for 90 and 365 days were 71% and 36% respectively. For 90- and 365-day survival, the Karnofsky performance score, muscle density and primary tumour were independently significantly associated. The visceral or subcutaneous fat area and their ratio and sarcopenia were not independently associated with overall survival.
Conclusions: Of the body morphology, only muscle density was statistically significant associated with overall survival after 90 and 365 days in patients with spinal bone metastases. Body fat distribution was not significantly associated with overall survival.
Introduction
The overall survival of cancer patients has increased, due to early detection and improved treatment [Citation1]. Because of this increased survival, more patients develop metastases, with metastases in the skeleton being the predominant site [Citation2]. Most bone metastases are located in the spinal column, where they can cause pain, deformity, fracture, spinal instability and neurological deficits [Citation3,Citation4]. In patients with symptomatic spinal metastases, radiation therapy with or without surgical stabilisation is often necessary [Citation3]. When the life expectancy of a patient is less than three months, the quality of life is generally considered to be hampered too much by the time needed for recovery and revalidation to justify the procedure [Citation5,Citation6]. Therefore, to determine the optimal treatment for individual patients, appropriate estimation of expected survival is necessary beforehand, as a patient might not benefit from a demanding intervention [Citation5–7].
At this moment, patient survival is estimated using clinical factors such as primary tumour biology, the presence of visceral/brain metastases and (preoperative) performance scores, but the prognostic value of these factors combined is moderate [Citation6,Citation8]. Evidence on other factors such as nutritional status as prognostic factors (e.g., biochemical markers, weight or BMI) is still limited [Citation9,Citation10]. In recent years, an increasing number of studies have focussed on body composition as a new and promising parameter for predicting prognosis in patients with malignancies [Citation11–13]. Body composition refers to the distribution of visceral and subcutaneous fat, obtained from information on axial CT-slices at the level of the third lumbar vertebra (L3) and also includes muscle area and muscle density [Citation13–15].
The aim of this study was to evaluate whether difference in body composition, including visceral fat area, subcutaneous fat area, total muscle mass using the skeletal muscle index and muscle density, were associated with survival in patients with spinal metastases.
Patients and methods
Patients were selected from a prospective cohort which included all patients receiving radiotherapy for bone metastases at a single centre since June 2013. All patients signed informed consent for the use of their clinical baseline and follow-up data, including self-reported quality of life and pain scores. The study protocol was approved by the Institutional Review and Ethics Board of our hospital. For this study, all patients who were treated with radiotherapy only for thoracic or lumbar spinal bone metastases between June 2013 and December 2016 were included. There was no distinction on radiotherapy scheme or modality as this did not influence patients’ overall survival. In the same way, no distinction was made on concurrent (systemic) therapy at inclusion. Patients’ medical records were used to collect patient characteristics. Characteristics included the Karnofsky Performance Score(KPS) to estimate general condition and the Charlson Comorbidity Index(CCI) to take medical history into account [Citation16]. For retrieving a patient’s vital status, a governmental database was used.
CT-measurements
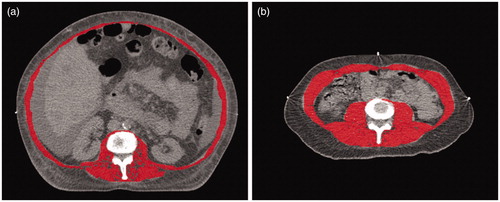
For all patients, routine radiotherapy treatment planning CT scans were performed, using a 16-detector row CT scanner (Brilliance, Philips Medical Systems, Eindhoven, The Netherlands). Images were reconstructed at a slice thickness of 3 mm. A single trained observer, blinded to the clinical information of the patients, performed all body morphology measurements. The reproducibility of these measurements has proved to be very high [Citation17]. One transverse CT image of the inferior surface of the L3 vertebral body was selected to manually delineate the abdominal muscle wall with VolumeTool, an in-house developed delineation tool to help radiotherapy treatment planning [Citation18]. Delineation of the abdominal muscle wall included the psoas, erector spinae, quadratus lumborum, transversus abdominus, external and internal oblique and rectus abdominus muscles. The subcutaneous fat area (SFA), visceral fat tissue area (VFA), total muscle area and muscle density were measured using tissue-specific absolute Hounsfield units (HU) thresholds [Citation19,Citation20]. To determine muscle density, the mean HU of the muscle area was measured. Decreased muscle density is an indicator for an increased lipid concentration in the skeletal muscle and is a known proxy for decreased muscle function [Citation12]. For the measurements of skeletal muscle, HUs from −29 to +150 were used, for subcutaneous and intra-muscular fat the value used ranged from −190 to −30 and for visceral fat the value ranged from −150 to −50 ( and ) [Citation2]. Subsequently, the VFA/SFA ratio was calculated by the simple division of the values for VFA and SFA. The skeletal muscle index was calculated by dividing the total muscle area by the square of the patient’s height in metres. The cut-off values for sarcopenia were <52.4 cm2/m2 for males and <38.5 cm2/m2 for females [Citation14,Citation21].
Figure 1. Example of a CT-analysis. (a) base CT-scan (b) total muscle area measurement (c) subcutaneous fat area measurement (d) visceral fat area measurement.
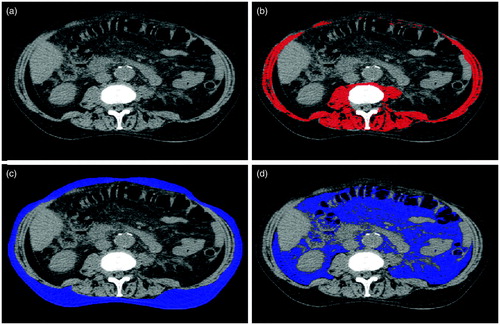
Figure 2. Example of muscle density. (a) patient with low muscle density. (b) patient with high muscle density.
Statistical analysis
Continuous data were presented as mean with standard deviation (SD) for normally distributed continuous variables and median with interquartile range (IQR) for not normally distributed and imputed continuous variables. Normality was tested using the Shapiro-Wilk test. Categorical data are presented as counts with percentages.
Survival was defined as days between start of radiation therapy and date of death from all causes, or end of follow-up on 31st of March 2018. There was no loss to follow up due to the use of the up to date governmental database. As some data were included retrospectively, missing data were analysed for patterns of randomness, imputation was done with multiple imputation using the Markov Chain Monte Carlo method. Results of the imputation were checked using convergence plots. The KPS was analysed as a score from 1–10. Using imputed data, univariable Cox regression analysis was performed to compute mortality hazard ratios with 95% confidence interval (95% CI). A multivariable Cox regression was performed to adjust for factors associated with outcome in univariable analysis for survival after 90 and 365 days. Patients were censored after 90 and 365 days for the corresponding analysis. When using categorical variables, the largest group within that variable was used as reference group. Before multivariable analysis, collinearity was tested using the Variance Inflation Factor(VIF) as well as proportionality assumptions for the Cox regression analysis [Citation22,Citation23]. Variables were excluded if the VIF was >10 and reconsidered with VIF > 5 [Citation22]. Statistical analyses were performed using SPSS, IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp [Citation20].
Results
Study population
A total of 310 patients with spinal metastases treated with palliative radiation therapy was included. Median follow-up was 202 days (IQR 73–576) and overall survival rates after 90 and 365 days were 71% and 36% respectively. The majority of patients was male (63%) (). The most common primary tumour originated from the lung (28%), followed by prostate and breast (27% and 18% respectively). Non-osseous metastases were present in 152 patients (49%), 22% of all patients had liver metastases and 3% had brain metastases. In 9% of the patients, neurological symptoms as a result of epidural compression of the spinal cord/cauda equina/nerve roots were present. Of these patients, 18 (6%) had ASIA-scale grade D, 7 (2%), 2 (0.6%), 1(0.3%) had ASIA scale C, B and A respectively [Citation24]. A minority of the patients (n = 115, 37%) received concurrent systemic therapy. There was no collinearity between any variables, as all VIFs were <5. Partial residuals using the Schoenfeld residuals method showed a linear relationship between residuals and continuous data. Missing data was found in 180 patients (58%), the majority of missing cases was found in the Karnofsky performance score (n = 134, 43%) and/or the patient’s height (n = 93, n = 30%) which is necessary to determine sarcopenia. Comparison between patients with and without at least one missing value can be found in Supplementary Table 1. Supplementary Figures 1 and 2 show the convergence plots of the imputation of the KPS and height.
Table 1. Imputed and original baseline characteristics of all patients with thoracic or lumbar bone metastases.
Univariable analysis showed that an increased age, increased VFA/SFA-ratio, increased SFA or a decrease in muscle density and a diagnosis of sarcopenia increased the probability of death. A higher KPS and having breast or prostate cancer as primary tumour compared with cancer of the lung decreased the probability of death ().
Table 2. Univariable Cox proportional hazard analysis for the risk of death after radiotherapy for bone metastases using pooled imputed data.
In a multivariable analysis for survival at 90 days, decreased muscle density was associated with a decreased survival at 90 and 365 days, adjusted for clinical factors such as KPS and primary tumour type; HR 0.62 (95% CI 0.41–0.94) and 0.70 (95% CI 0.53–0.92), respectively (). The subcutaneous fat area and fat ratio, as well as the presence of sarcopenia, were not statistically significant associated with overall survival at any time point.
Table 3. Multivariable Cox proportional hazard analysis for the risk of death after radiotherapy for bone metastases using pooled imputed data.
Discussion
This study aimed at investigating the association between body composition and overall survival in patients with spinal metastases treated with radiation therapy. To our knowledge, this is the first study addressing the impact of body composition in patients who receive palliative radiotherapy for spinal metastases. In this study, we found that muscle density was significantly associated with overall survival at 3 months and 1 year, adjusted for Karnofsky performance score (KPS), Charlson comorbidity index (CCI) and primary tumour. We did not find a statistically significant association between subcutaneous fat area (SFA), visceral fat area (VFA) or fat ratio (VFA/SFA) and survival of patients with spinal metastases receiving radiotherapy.
In previous studies, the effect of SFA, VFA, TMA, muscle density and the VFA/SFA-ratio have been assessed for overall survival and progression-free survival. In some studies, an increased VFA, SFA and VFA/SFA-ratio was associated with improved survival [Citation25]. The general hypothesis proposed so far has been that patients with a high VFA and SFA are in a generally better condition because low volume of adipose tissue in patients is linked to cancer progression. However, other authors have reached opposite conclusions with their study results [Citation9,Citation11] arguing that worse survival in patients with increased VFA could be linked to the detrimental hormonal activity of adipose tissue [Citation11,Citation18]. The adipose tissue is known to produce vascular endothelial growth factor (VEGF), which is a recognised factor in tumour growth and tumoral angiogenesis [Citation11].
Patients with advanced cancer can suffer from cachexia, which is a systemic tissue-wasting process in which the patient loses fat, muscle tissue and muscle quality in the form of lower muscle density [Citation2,Citation26–29]. Cachexia could have a negative impact on overall survival, as the patient’s general condition decreases [Citation26,Citation28]. Sarcopenia, which could be part of cachexia, but is also a syndrome in itself, is generally used as the term for loss of muscle mass and function. Unfortunately, there is limited consensus on the cut-off value for sarcopenia [Citation21,Citation30]. In our study, we used the cut-off value described by Prado et al. which is widely used [Citation21]. In accordance with our results, Okumura et al. reported that a decrease in muscle density was associated with decreased overall survival of patients after resection of pancreatic cancer in 301 patients, and Nattenmüller et al. reported the same correlation in 200 patients with lung cancer having received chemotherapy. Furthermore, the association between a decrease in muscle density and decreased survival was also reported for multiple other primary tumours [Citation21,Citation31,Citation32]. Similarly, in the study of Chambard and coworkers [Citation29] sarcopenia was also associated with worse outcome for patients with lung cancer and synchronal bone metastases. However, sarcopenia was not independently statistically significant associated with overall survival in primary, operable gastrointestinal cancers [Citation33]. The present study is the first study concerning patients with spinal metastases and did not focus on, or select one specific primary tumour [Citation1]. The study of Chambard et al. only took into account patients with synchronous bone metastases from lung carcinoma. Decreased muscle strength is a sign of poor prognosis and could be caused by low muscle density. In the present work we found an association between low muscle density and poor prognosis which was independent of other clinical factors as found in different studies as well [Citation2,Citation34]. Shachar et al. performed a meta-analysis to look at the prognostic value of sarcopenia on overall survival in patients with solid tumours [Citation21]. Contrary to our results, they found a significant difference in patients with and without sarcopenia in the multivariable analysis. In this study, we did find a significant difference in the univariable analysis, but not in the multivariable analysis. This could be due to the general condition of the cohort, as these are all patients with advanced metastatic cancer receiving palliative care.
During treatment and/or progression of disease in patients with advanced cancer, their body composition might change as patients lose weight due to loss of fat or muscle tissue. This change in body composition was described in the review by Pamoukdjian et al., where it was found that 39% of cancer patients had pre-treatment sarcopenia [Citation35]. Neuromuscular impairment and its effects on mobility and function can also have a profound effect on muscle mass and strength [Citation36]. Neuromuscular impairment was present in 9% of our patients, which could have confounded the association of muscle density and survival, but multivariable analysis showed the association to be independent of neuromuscular impairment.
In this and other studies, a single pre-treatment scan was used to assess body morphology instead of scans at multiple points in time. Nattenmüller and co-workers and Tan and co-workers assessed the change in body composition over a period of time, both with a mean follow-up of 4.4 months [Citation27]. Nattenmüller et al. showed that decreasing weight and loss of muscle tissue after chemotherapy was associated with worse survival in 200 patients with lung cancer. This effect was not reported by Tan et al., which might be due to their limited number of patients (n = 44) [Citation27]. For future research, follow-up scans to assess change in body morphology over time can be considered, to analyse if changes in body morphology are associated with overall survival in patients with spinal metastases.
The revised Katagiri scoring system added laboratory outcomes to their original model, which includes C-reactive protein (CRP); lactate dehydrogenase (LDH); serum albumin; serum calcium corrected for albumin level; platelet count; and total bilirubin [Citation37]. In their prognostic model, they found abnormal or critical laboratory values (e.g., CRP ≥ 0.4 mg/dL, LDH ≥ 250 IU/L, or serum albumin <3.7 g/dL or platelet count <100,000/lL, serum calcium level ≥10.3 mg/dL, or total bilirubin ≥1.4) were associated with decreased survival. Kardhade et al. also included multiple laboratory data in their machine learning model [Citation38]. Nonetheless, the evidence of these models is still limited as there has not been an external validation yet. In addition, these laboratory values are limited or not available in patients who receive radiotherapy alone. In future modelling, the laboratory values might prove to be useful.
One of the limitations of this study is the missing data on KPS and patient height. Using multiple imputations, the missing data were imputed and pooled data were used for the single and multiple variable analyses, so all patients could be handled as complete cases. KPS still showed to be significantly associated with overall survival, independent of other factors. Next, the cohort used is heterogeneous, which makes it hard to create a model on the factors associated with overall survival in this patient group. But this is also a strong point of this study, as it does make the model more pragmatic. Lastly, only patients treated with radiotherapy were included. It could be useful in further studies to also include surgically treated patients.
Conclusion
Better prediction of survival in patients with spinal metastases is crucial to optimise their care. CT analysis is an easy-to-perform measurement, as recent chest/abdominal CT-scans are available for most cases and for all patients who receive radiotherapy [Citation19]. As previously found in other studies, Karnofsky performance score and primary tumour were statistically significant associated with overall survival in patients with spinal metastases treated with palliative radiotherapy. In addition, we found that muscle density was statistically significant associated with overall survival. A diagnosis of sarcopenia was associated with overall survival in the univariable analysis, but the association was not independently statistically significant. Pre-treatment (planning) CT-scan analysis may provide useful information which can contribute to better care. We conclude that an analysis of body fat distribution and sarcopenia can improve predictions of overall survival, and suggest that these measurements are of value for future clinical multifactorial prediction models for this category of patients.
Supplemental Material
Download MS Word (99.3 KB)Acknowledgments
The authors would like to thank M.L. Groot Koerkamp for her help with the figures.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11(1):321–321.
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. JCO. 2013;31(12):1539–1547.
- Huisman M, van der Velden JM, van Vulpen M, et al. Spinal instability as defined by the spinal instability neoplastic score is associated with radiotherapy failure in metastatic spinal disease. Spine J. 2014;14(12):2835–2840.
- Kaloostian PE, Yurter A, Zadnik PL, et al. Current paradigms for metastatic spinal disease: an evidence-based review. Ann Surg Oncol. 2014;21(1):248–262.
- Laufer I, Sciubba DM, Madera M, et al. Surgical management of metastatic spinal tumors. Cancer Control. 2012;19(2):122–128.
- Verlaan JJ, Choi D, Versteeg A, et al. Characteristics of patients who survived < 3 months or > 2 years after surgery for spinal metastases: can we avoid inappropriate patient selection? J Clin Oncol. 2016;34(25):3054–3061.
- Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91(6):1503–1516.
- Bollen L, Wibmer C, Van der Linden YM, et al. Predictive value of six prognostic scoring systems for spinal bone metastases: an analysis based on 1379 patients. Spine. 2016;41(3):E155–162.
- Okamura A, Watanabe M, Mine S, et al. Clinical impact of abdominal fat distribution on prognosis after esophagectomy for esophageal squamous cell carcinoma. Ann Surg Oncol. 2016;23(4):1387–1394.
- Ebadi M, Martin L, Ghosh S, et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. 2017;117(1):148–155.
- Grignol VP, Smith AD, Shlapak D, et al. Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy. Surg Oncol. 2015;24(4):353–358.
- Yip C, Dinkel C, Mahajan A, et al. Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging. 2015;6(4):489–497.
- Antoun S, Lanoy E, Iacovelli R, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 2013;119(18):3377–3384.
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635.
- van Vugt JLA, Gaspersz MP, Vugts J, et al. Low skeletal muscle density is associated with early death in patients with perihilar cholangiocarcinoma regardless of subsequent treatment. Dig Surg. 2018;36(2):144–152.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Irlbeck T, Massaro JM, Bamberg F, et al. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes. 2010;34(4):781–787.
- Bol GH, Kotte ANTJ, van der Heide UA, et al. Simultaneous multi-modality ROI delineation in clinical practice. Comput Methods Programs Biomed. 2009;96(2):133–140.
- Mourtzakis M, Prado CMM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006.
- IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.
- Shachar SS, Williams GR, Muss HB, et al. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67.
- O'Brien R. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–690.
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241.
- Roberts TT, Leonard GR, Cepela DJ. Classifications in brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin Orthop Relat Res. 2017;475(5):1499–1504.
- Mizuno R, Miyajima A, Hibi T, et al. Impact of baseline visceral fat accumulation on prognosis in patients with metastatic renal cell carcinoma treated with systemic therapy. Med Oncol. 2017;34(4):47.
- Camus V, Lanic H, Kraut J, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur J Haematol. 2014;93(1):9–18.
- Tan BH, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15(22):6973–6979.
- Rutten IJ, Ubachs J, Kruitwagen RF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017;43(4):717–724.
- Chambard L, Girard N, Ollier E, et al. Bone, muscle, and metabolic parameters predict survival in patients with synchronous bone metastases from lung cancers. Bone. 2018;108:202–209.
- Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med. 2016;31(4):643–650.
- Ha Y, Kim D, Han S, et al. Sarcopenia predicts prognosis in patients with newly diagnosed hepatocellular carcinoma, independent of tumor stage and liver function. Cancer Res Treat. 2018;50(3):843–851.
- Rier HN, Jager A, Sleijfer S, et al. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. The Breast. 2017;31:9–15.
- Black D, Mackay C, Ramsay G, et al. Prognostic value of computed tomography: measured parameters of body composition in primary operable gastrointestinal cancers. Ann Surg Oncol. 2017;24(8):2241–2251.
- Friedman J, Lussiez A, Sullivan J, et al. Implications of sarcopenia in major surgery. Nutr Clin Pract. 2015;30(2):175–179.
- Pamoukdjian F, Bouillet T, Levy V, et al. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2018;37(4):1101–1113.
- Tournadre A, Vial G, Capel F, et al. Sarcopenia. Joint Bone Spine. 2019;86(3):309–314.
- Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3(5):1359–1367.
- Karhade AV, Shin JH, Schwab JH. Prognostic models for spinal metastatic disease: evolution of methodologies, limitations, and future opportunities. Ann Transl Med. 2019;7(10):219.
