Background and rationale
The treatment of locally advanced head and neck squamous cell carcinoma (LAHNSCC) in Denmark has improved significantly during the recent decades [Citation1–3] due to a gradually developed treatment strategy based on continuous clinical trials performed by the Danish Head and Neck Cancer Group (DAHANCA). As a result, the current national DAHANCA guidelines prescribe IMRT-based radiotherapy (RT) with accelerated fractionation (66–68 Gy in 33–34 fractions, six fractions per week) together with the hypoxic modifier nimorazole and weekly cisplatin. After such treatment, some 80% of the patients are without recurrence at 3 years of follow-up. The prognosis, however, remains poor for a smaller group of patients with LAHNSCC and human papilloma virus-infection (HPV)/p16-negative tumors [Citation4].
Tumor hypoxia causes a relative resistance to radiation treatment [Citation5,Citation6]. It can be identified non-invasively by PET/CT using hypoxia specific tracers [Citation7], like 18F-fluoroazomycine arabinoside (FAZA), but hypoxia imaging is not used routinely in the clinical work-up or RT planning. However, the DAHANCA 24 trial demonstrated a significantly worse disease-free survival at 30 months of follow-up for patients PET-imaged with hypoxic HNSCC before the initiation of definitive (chemo-)radiation [Citation8]. This result was demonstrated despite the use of the hypoxic radio-sensitizing drug nimorazole [Citation8], and patients with HPV/p16-negative tumors had the most unfavorable prognosis, as more than one-third of these patients suffered a recurrence [Citation9].
An increased dose of irradiation to the tumor is likely to provide a higher curability. Such increase can be achieved by applying a higher total dose given in smaller doses per fraction (hyperfractionation) due to a better therapeutic ratio between tumor and normal tissues [Citation10–13]. To maintain an accelerated overall treatment time, the hyperfractionated treatment must be given in multiple daily fractions. To intensify further, systemic treatment with nimorazole and cisplatin can be added. Such therapeutic strategy is supported by recent large meta-analyses [Citation14–17].
The feasibility of introducing intensified, dose-escalated RT to high-risk, stage III–IV patients has recently been evaluated in the DAHANCA 28 trial [Citation18], where the total dose to the gross tumor volume (GTV) was increased to 76 Gy by hyperfractionated, accelerated treatment with concomitant daily hypoxic cell sensitizer, nimorazole, and weekly low-dose cisplatin (HART-CN). It proved to be tolerable for HPV/p16-negative HNSCC patients in good general health.
Aims and hypothesis
The DAHANCA 33 trial combines the imaging methodology of the DAHANCA 24 trial with the treatment modality of the DAHANCA 28 trial and investigates whether a dose escalation with HART-CN improves the curability of HPV/p16-negative HNSCC patients selected by hypoxia imaging. Thus, the study will test the hypothesis that dose escalation by HART-CN is acceptable and improves curability of radiotherapy in HNSCC patients characterized by having locally advanced, FAZA-hypoxic tumors.
Methods
Study design and setting
The DAHANCA 33 trial is an open, prospective, non-randomized, phase II multi-center trial investigating the effect of a moderate dose increase on the probability of achieving 3-year tumor control for patients treated for hypoxic, high-risk HNSCC. It is investigator-initiated on behalf of DAHANCA and open to recruit participants from all Danish centers offering HART-CN. The trial is coordinated from the DAHANCA secretariat situated at the Department of Experimental Clinical Oncology, Aarhus University Hospital. The complete protocol can be found at the DAHANCA website: www.dahanca.dk.
Participants, eligibility criteria and recruitment
Patients with biopsy-proven, locally advanced, non-metastatic, non-resected squamous cell carcinoma of the larynx, pharynx and oral cavity (UICC stages III–IV) are eligible. For oropharynx cancers, the routinely performed immunostaining for p16-expression must be negative. Patients must be in good overall condition with a WHO performance status of 0–1.
Image acquisition and scan protocols
Patients will undergo a FAZA PET/CT during treatment planning to establish the hypoxic status. Two hours after injection of 400 MBq (± 10%) 18F-FAZA, non-fasting patients are scanned statically in 1–2 bed positions for 10–20 min in treatment position fixed in a molded mask using alignment of lasers. Tracer emission data are corrected for attenuation, scatter and random coincidences following iterative reconstruction. A NEMA phantom is used for calibration and best possible standardization of scans between different centers.
MIM® software (MIM® Software Inc., Cleveland, OH, USA) or similar is used for image interpretation (). The attenuation CT of the FAZA PET/CT is co-registered and deformable fused with the planning CT, allowing for the GTV to be transferred to the FAZA PET/CT. The hypoxic status of the tumor and involved lymph nodes is established using previous methods used in the DAHANCA 24 trial [Citation8,Citation9]. The mean uptake of FAZA in a manually delineated volume of deep neck muscle in each individual patient serves as reference tissue. A tumor-to-muscle threshold will be used to automatically delineate the hypoxic volume of the GTVtumor and GTVnode. Any hypoxic volume (minimum the size of a single voxel) will group the tumor as ‘hypoxic’.
Figure 1. FAZA PET/CT of a patient with a T2N2b HPV/p16neg oropharyngeal cancer. The axial and sagittal slices of the attenuation CT of the FAZA PET are displayed with the gross tumor volume (GTV) in red. Inside the GTV, voxels above hypoxic threshold are contoured in yellow. On the sagittal slices, the delineated muscle reference is shown (blue contour). The hypoxic volume on the pre-treatment, baseline FAZA PET/CT in seen in (A) and after ∼20 Gy is given (B). The hypoxic volume has increased during treatment, but will be covered by the high dose, as the dose escalation is applied to the entire GTV in the DAHANCA 33 trial.
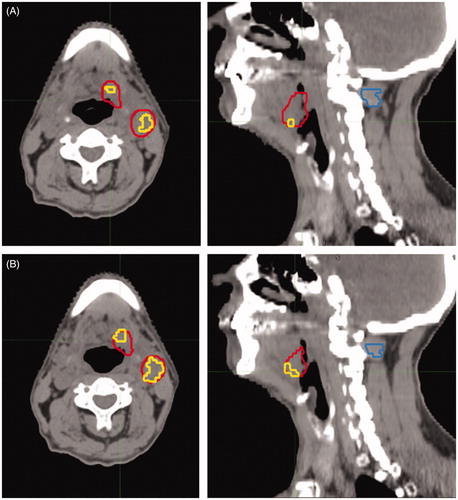
During the treatment, if possible after 17–20 of 56 fractions are given, an additional FAZA PET/CT scan will be performed. Image acquisition, scan protocol, reconstruction and image interpretation are as described for the baseline FAZA PET/CT.
Treatment intervention, modification and adherence
The model of intervention is a single group assignment with one experimental study arm. Patients are allocated for HART-CN if the GTV (primary tumor or involved node) contains a hypoxic volume. Lymph nodes are considered involved if PET-positive and/or enlarged. Target volumes and normal tissue are delineated as described in the DAHANCA national guidelines (www.dahanca.dk). In short, HART is applied as a prescribed dose to the CTV1 (typically GTV + 5 mm margin) of 76 Gy in 56 fractions, 1.36 Gy per fraction, 10 fractions weekly, delivered as simultaneous integrated boost intensity modulated radiotherapy (SIB-IMRT). Two daily fractions are given with at least 6 h in between fractions. High risk anatomical areas including the clinical target volume (CTV1) plus a 5 mm isotropic margin are given 66 Gy in total dose, 1.16 Gy per fraction, and elective areas including uninvolved lymph nodes are treated to a total of 56 Gy with 1 Gy per fraction. All patients will receive daily nimorazole (1.2 g/m2), and node-positive patients will in addition receive systemic chemotherapy, cisplatin (40 mg/m2, max 70 mg per cycle) weekly. Patients unfit for concurrent chemotherapy are allowed a modified regime without chemotherapy.
Dose reductions in cisplatin and/or nimorazole are allowed and recommended if patients experience intolerable adverse effects.
Follow up and endpoints
The patients are followed with clinical examinations with regular intervals for up to 36 months after completed RT. At 3 months, a routine MRI and/or PET/CT of the head and neck region is performed to assess the overall treatment result. Elective neck dissections are not allowed. Imaging (MRI or PET/CT) is repeated again at any time point, if a recurrence is suspected.
The primary endpoint is loco-regional tumor control in the primary tumor site (T-site) and in the lymph nodes of the neck (N-site) at 3 years of follow-up. Recurrences must be verified by biopsy. Salvage surgery should be performed if possible.
Imaging of the recurrence will be fused using deformable image registration with the planning CT to evaluate the site of recurrence and the dose given to the site of recurrence. Furthermore, it will be deformable fused with the pre-treatment FAZA PET/CT scan to investigate the spatial relationship to the hypoxic sub-volume.
Secondary endpoints are disease-free survival, overall survival, acute and late toxicity, localization of hypoxia in T- and N-site before and during treatment.
Data collection and statistical considerations
Data on the baseline characteristics of the patients, the parameters of the FAZA PET/CT imaging, the treatment prescribed and given, the toxicity during treatment and the outcome after treatment are entered prospectively in the DAHANCA database.
The DAHANCA 33 trial aims to include 60 patients with baseline hypoxia. The trial is a phase II superiority study and the experimental dose escalation arm is compared to a similar cohort of patients from the previous DAHANCA 24 study. With approximately 60 patients included and a loco-regional control rate expected to be similar to that of the DAHANCA 28 trial, a 20% increase in loco-regional control in the DAHANCA 33 trial should be detectable with a power of 80% and an alpha of 5%.
The Kaplan–Meier estimate is used for survival analysis, and the log-rank test is applied. Loco-regional failure rate is analyzed with correction for competing events. Binary parameters are compared by the chi-square test or Fishers exact test.
Quality assurance of treatment plans
The DAHANCA quality assurance (QA) group will evaluate all treatment plans to ensure adherence to the DAHANCA radiotherapy guidelines [Citation19]. The QA items are among others: dose prescription, minimum dose to the clinical target volume (CTV1), maximum dose to the spinal cord and brain stem and the overall treatment length.
Perspectives
The idea of increasing the dose to a volume containing a treatment resistant tumor cell population seems reasonable from a radiobiological view point; however, the clinical evidence that such dose increase will improve loco-regional control in hypoxic tumors is limited [Citation20]. Furthermore, the best strategy of hypoxia-based treatment intensification is unsettled. Ongoing trials studying this matter are using different approaches; by dose escalation to the entire GTV (e.g., The Escalox Trial, NCT01212354 [Citation21]) or selectively to the hypoxic areas within the GTV (‘dose-painting’, e.g., Zips and coworkers, NCT02352792 [Citation20]). A major cause for concern in terms of boosting dose only to the hypoxic sub-volumes is the sharp (non-biological based) cut-point in the definition of hypoxia, and that these hypoxic areas could show geographical shifts before and during radiotherapy [Citation22–25].
demonstrates that a dose escalation to the entire GTV will eliminate all uncertainties related to the temporal and geographical stability of a hypoxic-resistant tumor sub-volume, and any failure to show an improved tumor control will not be rooted in a scenario of ‘missing the target’. When an actual risk reduction is demonstrated for this particular patient group selected by functional imaging, then the next step of focusing on selective dose escalation to only the resistant parts of the GTV (dose painting) seems warranted in terms of reducing toxicity of the treatment.
The DAHANCA 33 study will contribute to the existing knowledge of the value of using hypoxia imaging for selection of patients with poor prognosis for dose-escalated treatment. This will allow a more individualized selection of patients for such intensified and harsh treatment. Thus, the study will hopefully demonstrate whether or not a moderate dose increase will improve curability in high-risk patients with LAHNSCC, for whom standard treatment often fails.
Ethics approval
The DAHANCA 33 trial is performed according to the WHO Helsinki Declaration and is approved by the relevant ethical committee (file no. 1-10-72-284-16) and registered with the national Data Protection Agency (file no. 1-16-02-655-16). The trial is registered at ClinicalTrials.gov with Identifier NCT02976051. The protocol attempts to adhere to the SPIRIT guidelines (see checklist in Supplementary Material).
Supplemental Material
Download PDF (138.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Overgaard J, Jovanovic A, Godballe C, et al. The Danish Head and Neck Cancer database. Clin Epidemiol. 2016;8:491–496.
- Baumann M, Krause M, Overgaard J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16(4):234–249.
- Overgaard J. Improving radiotherapy of squamous cell carcinoma of the head and neck (HNSCC) through a continuous process of biological based clinical trials − a 40-year experience from the Danish Head and Neck Cancer Group – DAHANCA. Eur J Cancer. 2017;72:S102.
- Lassen P, Lacas B, Pignon JP, et al. Prognostic impact of HPV-associated p16-expression and smoking status on outcomes following radiotherapy for oropharyngeal cancer: the MARCH-HPV project. Radiother Oncol. 2018;126(1):107–115.
- Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25(26):4066–4074.
- Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77(1):18–24.
- Horsman MR, Mortensen LS, Busk M, et al. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9(12):674–687.
- Mortensen LS, Johansen J, Kallehauge J, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105(1):14–20.
- Saksø M, Mortensen L, Primdahl H, et al. OC-0268: FAZA PET hypoxia as a marker of loco-regional recurrence in HNSCC? Results from the DAHANCA 24 trial. Radiother Oncol. 2018;127:S136.
- Peters LJ, Ang KK. The role of altered fractionation in head and neck cancers. Semin Radiat Oncol. 1992;2(3):180–194.
- Saksø M, Andersen E, Bentzen J, et al. A prospective, multicenter DAHANCA study of hyperfractionated, accelerated radiotherapy for head and neck squamous cell carcinoma. Acta Oncol. 2019;58(10):1495–1501.
- Evensen JF, Sand Hansen H, Overgaard M, et al. DAHANCA 9 – a randomized multicenter study to compare accelerated normo-fractionated radiotherapy with accelerated hyperfractionated radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC). Acta Oncol. 2019;58(10):1502–1505.
- Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368(9538):843–854.
- Lacas B, Bourhis J, Overgaard J, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18(9):1221–1237.
- Budach W, Hehr T, Budach V, et al. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer. 2006;6(1):28.
- Blanchard P, Landais C, Lacas B, et al. Update of the meta-analysis of chemotherapy in head and neck cancer (MACH-NC). Radiother Oncol. 2017;122:9.
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol. 2011;100(1):22–32.
- Saksø M, Jensen K, Andersen M, et al. OC-041 DAHANCA 28a: phase I/II study of acc. hyperfractionated RT, cisplatin and nimorazole in P16-LAHNSCC. Radiother Oncol. 2019;132:21–22.
- Hansen CR, Johansen J, Kristensen CA, et al. Quality assurance of radiation therapy for head and neck cancer patients treated in DAHANCA 10 randomized trial. Acta Oncol. 2015;54(9):1669–1673.
- Welz S, Mönnich D, Pfannenberg C, et al. Prognostic value of dynamic hypoxia PET in head and neck cancer: results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother Oncol. 2017;124(3):526–532.
- Pigorsch SU, Wilkens JJ, Kampfer S, et al. Do selective radiation dose escalation and tumour hypoxia status impact the loco-regional tumour control after radio-chemotherapy of head & neck tumours? The ESCALOX protocol. Radiat Oncol. 2017;12:45.
- Stieb S, Eleftheriou A, Warnock G, et al. Longitudinal PET imaging of tumor hypoxia during the course of radiotherapy. Eur J Nucl Med Mol Imaging. 2018;45(12):2201–2217.
- Ureba A, Lindblom E, Dasu A, et al. Non-linear conversion of HX4 uptake for automatic segmentation of hypoxic volumes and dose prescription. Acta Oncol. 2018;57(4):485–490.
- Busk M, Horsman MR, Jakobsen S, et al. Imaging hypoxia in xenografted and murine tumors with 18F-fluoroazomycin arabinoside: a comparative study involving microPET, autoradiography, PO2-polarography, and fluorescence microscopy. Int J Radiat Oncol Biol Phys. 2008;70(4):1202–1212.
- Skjøtskift T, Evensen ME, Furre T, et al. Dose painting for re-irradiation of head and neck cancer. Acta Oncol. 2018;57(12):1693–1699.
