Abstract
Introduction: Proton beam therapy (PBT) reduces normal organ dose compared to intensity-modulated radiation therapy (IMRT) for patients with major salivary gland tumors. It is not known whether this dosimetric advantage is clinically meaningful for reducing acute toxicity.
Methods: We evaluated treatment parameters and acute toxicity outcomes of patients with major salivary gland cancers enrolled on the Proton Collaborative Group REG001-09 trial (NCT01255748).
Results: One-hundred and five patients with a median age of 61 years were included. The majority had parotid (N = 90) versus submandibular gland (N = 15) tumors. The patients were treated across seven institutions in the United States between 2010 and 2017, most commonly in the postoperative setting (70.5%) although a minority were treated definitively (29.5%). Median PBT dose was 66.5 GyE in 33 fractions; only one patient was prescribed less than 50 GyE. Chemotherapy was given concurrently to 20%. Median follow-up was 14.3 months. Acute grade 2 or higher toxicity included nausea (1.5%), dysgeusia (4.8%), xerostomia (7.6%), mucositis (10.5%) and dysphagia (10.5%).
Conclusions: PBT should be strongly considered when ipsilateral radiation therapy is indicated for major salivary gland cancer based on a considerably lower incidence of acute grade 2 or higher toxicity in this analysis compared to historical IMRT outcomes.
Introduction
Salivary gland tumors (SGTs) are uncommon, representing less than about 5% of all cancers of the head and neck [Citation1]. Surgery is a mainstay of care although radiation therapy (RT) also plays an important role in the postoperative setting of high-risk features (e.g., close/positive margin, advanced T stage, lymph node involvement) and if the tumor is unresectable [Citation2–6].
Although target volumes for major SGTs typically are limited to the involved side of the head and potentially neck, acute toxicity from highly conformal X-ray techniques like intensity-modulated radiation therapy (IMRT) is not trivial [Citation7,Citation8]. Over 50% of the patients treated with IMRT may experience at least grade 2 toxicity including dysgeusia and/or mucositis that can negatively affect the quality of life [Citation9]. These adverse events are related to both low and high dose that is unintentionally delivered to normal tissues of the head and neck [Citation10].
While it is unavoidable that some dose is delivered distal to the target within the path of an X-ray beam, there is essentially no exit dose within a proton beam. Thus, large volumes of the head and neck are spared from any radiation – even low dose – with proton beam therapy (PBT) as compared to IMRT, especially considering that the target volumes for SGT patients are typically unilateral [Citation11–13]. There is a lack of published data to indicate whether the dosimetric advantage of PBT versus IMRT is clinically meaningful.
Material and methods
We reviewed a multi-institutional database of patients treated with PBT on the Proton Collaborative Group (PCG) REG001-09 trial (NCT01255748) who received potentially curative PBT therapy for major SGTs. This registry study was designed to capture prospectively recorded outcomes of PBT delivered according to institutional standard of care; a particular PBT planning or treatment delivery approach was not designated by the study protocol. As with any clinical trial, study participation was optional and patients were required to sign informed consent for enrollment. The patients who received PBT either postoperatively or definitively were included although those treated with palliative intent, including those who underwent reirradiation, were not included. Chemotherapy was delivered at the discretion of the treating physician.
Of 717 total patients with head and neck cancer enrolled to the registry trial, 105 patients with major SGTs who met inclusion criteria were included in this analysis. These patients were treated across seven institutions between 2010 and 2017.
All the patients underwent CT simulation in the supine position with a customized 3- or 5-point thermoplastic mask to facilitate daily reproducibility of treatment setup. Intravenous (IV) contrast was recommended to facilitate target volume delineation. The gross tumor volume (GTV) included visible disease as seen on diagnostic imaging studies. The clinical target volume (CTV) included the GTV or tumor bed in the adjuvant setting and areas of potential microscopic disease spread. Elective treatment of high-risk lymph node regions in the ipsilateral neck was optional. Perineural invasion of named nerves was an indication for treating the involved nerves to the base of skull. All the patients were treated to unilateral targets. Planning target volume (PTV) margins were typically 3–5 mm from the CTV.
Although there was some heterogeneity in PBT dose prescription across institutions, 70 GyE in 35 fractions was typically prescribed to areas of gross disease, 66 GyE in 33 fractions to microscopically positive margins, 60 GyE in 30 fractions to the postsurgical tumor bed, and 54 GyE in 27 fractions to lower risk areas including elective cervical lymph nodes. PBT was commonly delivered using 2–4 fields. Beam-specific PTV margins consisting of 3% Hounsfield unit density uncertainty plus 1–3 mm were used to account for range uncertainty. Daily image guidance was achieved using orthogonal X-ray scans as well as with cone-beam CT scans, which was available at a minority of the treating institutions.
Normal organ constraints varied between participating institutions, but generally included the following: contralateral parotid gland mean <10 Gy, contralateral submandibular gland mean <20 Gy, oral cavity mean <30 Gy, larynx mean <45 Gy, spinal cord maximum 45 Gy, brainstem maximum 45 Gy, cochlea V55 < 5%, optic nerve/chiasm maximum 54 Gy.
Acute toxicities were assessed per the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 criteria and were defined as being present during or within 3 months from the completion of PBT. All the toxicities were physician-reported at initial consultation (baseline), weekly during PBT, and at each follow-up visit. Follow-up was typically performed at 3-month intervals.
Results
Patient and tumor characteristics are summarized in . The median follow-up was 14.3 months from the completion of PBT (range 0.8–60.3) and the median age of patients at diagnosis was 61.1 years (range 9.7–96.3). Nearly all had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Parotid gland tumors comprised the majority (85.7%) of treated tumors with the rest involving the submandibular gland (14.3%). The most common histologies were squamous cell carcinoma (21%), mucoepidermoid carcinoma (21%), adenocarcinoma (11.4%) and adenoid cystic carcinoma (10%).
Table 1. Patient, tumor and treatment characteristics.
The median prescription was 66 GyE in 33 fractions using either uniform scanning (44.8%) or pencil beam scanning (33.3%). Most received PBT in the postoperative setting (70.5%) while the remainder received definitive therapy (29.5%). A minority received PBT to the ipsilateral neck (32.4%) or base of skull (6.7%). Most (80%) did not receive concurrent chemotherapy.
The worst acute grade 2 or grade 3 toxicities for each patient are listed in . Dermatitis was the most common grade 2 (58%) or grade 3 (11%) toxicity. The most frequent grade 2 toxicities other than dermatitis were esophagitis (13%), oral mucositis (11%) and fatigue (10%). The only grade 3 toxicities other than dermatitis were oral mucositis (3%), dysphagia (2%) and fatigue (1%). There was no significant difference in toxicity based on treatment with pencil beam scanning (PBS) or uniform scanning (US).
Table 2. Worst acute toxicity in patients treated with PBT for major salivary gland tumor.
Discussion
Radiation therapy for head and neck cancers is associated with significant acute toxicity that can lead to treatment breaks and compromise long-term tumor control. Even among the patients with major SGTs where treatment is only delivered to the ipsilateral head and neck, acute toxicity can be significant despite using highly conformal IMRT as a result of large volumes of normal tissue exposed to lower doses of radiation (i.e., <20 Gy) [Citation7].
Despite the normal tissue sparing achieved with PBT versus XRT for major SGT patients (), there are limited published data to confirm that these differences are meaningful [Citation13,Citation14]. A study from MD Anderson Cancer Center (MDACC) of 24 pediatric patients with SGT reported higher dose to multiple avoidance structures including the oral cavity (mean 20.7 Gy vs. 4.6 Gy; p < .05) and larynx (mean 44.3 Gy vs. 11.3 Gy; p < .05) [Citation14]. PBT was associated with less mucositis (91% vs. 46%; p < .05) and there was a trend toward reduced grade 2–3 dysphagia (27% vs 0%; p = .08). Investigators at Memorial Sloan Kettering Cancer Center (MSKCC) performed a similar analysis in 41 adult patients who received ipsilateral RT, and like the MDACC study also found a large difference in oral cavity dose favoring PBT (mean 20.6 Gy vs. 0.94 Gy; p < .001) that was associated with reduced grade 2 or higher dysgeusia (65.2% vs. 5.6%; p < .001) and mucositis (52.2% vs. 16.7%; p = .005) [Citation13]. PBT patients experienced less grade 2 nausea (56.5% vs. 11.1%; p = .003) that correlated with lower brainstem dose (maximum 29.7 Gy vs. 0.94 GyE; p < .001).
Figure 1. Improved normal tissue sparing with proton beam therapy (bottom) versus intensity-modulated radiation therapy (top) in a patient treated for a parotid gland tumor.
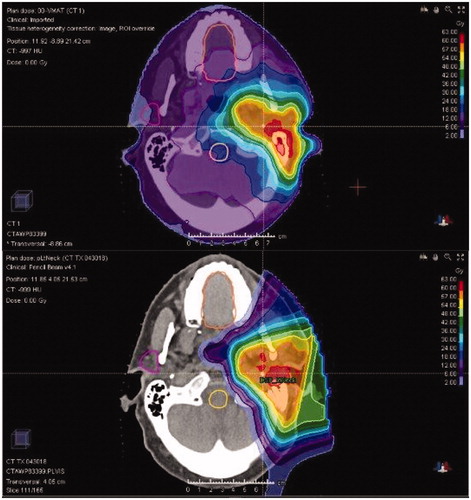
Our study represents the largest analysis of major SGT patients treated with PBT to date and supports the conclusions of the aforementioned publications that PBT achieves less moderate and severe acute toxicity than is expected from IMRT (). The similarity between our results and those from the MSKCC study, which includes a similar percentage of patients receiving concurrent chemotherapy, in particular, are striking: grade 2–3 mucositis (14% vs. 17%), dysgeusia (5% vs. 6%), and nausea (2% vs. 11%).
Table 3. Acute toxicity outcomes from IMRT and PBT in patients treated with PBT for major salivary gland tumor.
Although we were not able to perform a formal dosimetric analysis it is reasonable to expect that PBT achieved superior sparing of the brain and brainstem compared to what would have been delivered with IMRT, and that this contributed to our favorable toxicity findings [Citation13]. Kocak-Uzel et al. demonstrated that higher mean dose to the area postrema, brainstem, dorsal vagal complex, medulla oblongata, solitary nucleus, oropharyngeal mucosa and whole brain was significantly associated with clinically significant nausea and vomiting among 130 head and neck cancer patients treated with IMRT [Citation15]. In fact, the percent-volume of these structures receiving 20–40 Gy was more strongly associated with ematogenesis than higher doses. Several other publications have also demonstrated that reduced mean and low-dose exposure to substructures of the brain and brainstem minimizes the probability of not only ematogenesis [Citation16–19], but also potentially severe fatigue [Citation20–22]. Lastly, dysgeusia is among the most influential factors that negatively affects quality of life [Citation23] and has been significantly correlated with mean oral cavity dose [Citation9,Citation10], which is routinely <1 Gy with PBT compared to >20 Gy with IMRT among ipsilateral head and neck patients [Citation13].
Our finding that most patients treated with PBT experienced clinically significant dermatitis was not surprising given the superficial nature of the target volumes for this patient population and reduced skin sparing in this situation. The MSKCC group found that PBT resulted in a higher incidence of grade 2 or 3 dermatitis versus IMRT (PBT 100% vs. IMRT 73.9%; p = .032). This likely was due to the use of passive scattering or uniform scanning, which cannot achieve proximal dose conformality and thus is poor at skin sparing for superficial targets. In contrast, our study included a substantial percentage of patients treated with PBS that is much better able to limit proximal dose and achieve better skin sparing [Citation24]. This may explain why we observed a lower incidence of grade 2 or 3 dermatitis (69%) compared to the MSK study (100%). However, we did not detect a significant difference in any toxicity including dermatitis based on use of PBS or US among patients in our study. There are several potential confounders in our ability to accurately compare toxicities based on PBS versus US including unknown beam arrangement, use of bolus, and target volume characteristics. Whether PBS reduces toxicity in a meaningful way remains an outstanding question and warrants additional evaluation by others.
The strengths of this study include the prospectively recorded outcomes, multi-institutional participation and large number of patients. Limitations include the lack of detailed treatment planning and dosimetric information, and the heterogeneity in treatment approach that is inherently present in a multi-institutional study. However, it could be argued that this heterogeneity may better provide a more diverse ‘real world’ evaluation of outcomes. Lastly, there was a high percentage of squamous cell carcinoma represented in our study; we could not completely rule out that some if not all may have represented salivary gland metastases from a prior undocumented primary cutaneous malignancy, although none was recorded as such. Regardless, these patients were not excluded as we did not expect that they would influence our ability to evaluate our primary endpoint of acute toxicity.
Conclusions
We demonstrated a favorably low incidence of moderate and severe acute toxicities in the largest analysis of prospectively recorded acute toxicity outcomes among major SGT patients treated with PBT. Additional evaluation is needed with respect to late toxicities and tumor control outcomes. Our outcomes strengthen the rationale for an ongoing phase 2 randomized evaluation of PBT versus IMRT for unilateral head and neck cancer patients (NCT02923570).
Disclosure statement
The authors report no conflicts of interest.
References
- Sun EC, Curtis R, Melbye M, et al. Salivary gland cancer in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1095–1100.
- Orlandi E, Iacovelli NA, Bonora M, et al. Salivary gland. Photon beam and particle radiotherapy: present and future. Oral Oncol. 2016;60:146–156.
- Adelstein DJ, Koyfman SA, El-Naggar AK, et al. Biology and management of salivary gland cancers. Semin Radiat Oncol. 2012;22(3):245–253.
- Park G, Lee SW. Postoperative radiotherapy for mucoepidermoid carcinoma of the major salivary glands: long-term results of a single-institution experience. Radiat Oncol J. 2018;36(4):317–324.
- Mifsud MJ, Tanvetyanon T, McCaffrey JC, et al. Adjuvant radiotherapy versus concurrent chemoradiotherapy for the management of high-risk salivary gland carcinomas. Head Neck. 2016;38(11):1628–1633.
- Safdieh J, Givi B, Osborn V, et al. Impact of adjuvant radiotherapy for malignant salivary gland tumors. Otolaryngol Head Neck Surg. 2017;157(6):988–994.
- Schoenfeld JD, Sher DJ, Norris CM, Jr, et al. Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):308–314.
- Gebhardt BJ, Ohr JP, Ferris RL, et al. Concurrent chemoradiotherapy in the adjuvant treatment of high-risk primary salivary gland malignancies. Am J Clin Oncol. 2018;41(9):888–893.
- Deshpande TS, Blanchard P, Wang L, et al. Radiation-related alterations of taste function in patients with head and neck cancer: a systematic review. Curr Treat Options Oncol. 2018;19(12):72.
- Sapir E, Tao Y, Feng F, et al. Predictors of dysgeusia in patients with oropharyngeal cancer treated with chemotherapy and intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96(2):354–361.
- Owosho AA, Yom SK, Han Z, et al. Comparison of mean radiation dose and dosimetric distribution to tooth-bearing regions of the mandible associated with proton beam radiation therapy and intensity-modulated radiation therapy for ipsilateral head and neck tumor. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(5):566–571.
- Stromberger C, Cozzi L, Budach V, et al. Unilateral and bilateral neck SIB for head and neck cancer patients: intensity-modulated proton therapy, tomotherapy, and RapidArc. Strahlenther Onkol. 2016;192(4):232–239.
- Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118(2):286–292.
- Grant SR, Grosshans DR, Bilton SD, et al. Proton versus conventional radiotherapy for pediatric salivary gland tumors: acute toxicity and dosimetric characteristics. Radiother Oncol. 2015;116(2):309–315.
- Kocak-Uzel E, Gunn GB, Colen RR, et al. Beam path toxicity in candidate organs-at-risk: assessment of radiation emetogenesis for patients receiving head and neck intensity modulated radiotherapy. Radiother Oncol. 2014;111(2):281–288.
- Ciura K, McBurney M, Nguyen B, et al. Effect of brain stem and dorsal vagus complex dosimetry on nausea and vomiting in head and neck intensity-modulated radiation therapy. Med Dosim. 2011;36(1):41–45. Spring
- Lee VH, Ng SC, Leung TW, et al. Dosimetric predictors of radiation-induced acute nausea and vomiting in IMRT for nasopharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;84(1):176–182.
- Monroe AT, Reddy SC, Gibbs GL, et al. Factors associated with radiation-induced nausea and vomiting in head and neck cancer patients treated with intensity modulated radiation therapy. Radiother Oncol. 2008;87(2):188–194.
- Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):747–755.
- Gulliford SL, Miah AB, Brennan S, et al. Dosimetric explanations of fatigue in head and neck radiotherapy: an analysis from the PARSPORT Phase III trial. Radiother Oncol. 2012;104(2):205–212.
- Group MDAHNCSW. Fatigue following radiation therapy in nasopharyngeal cancer survivors: a dosimetric analysis incorporating patient report and observer rating. Radiother Oncol. 2019;133:35–42.
- Ferris MJ, Zhong J, Switchenko JM, et al. Brainstem dose is associated with patient-reported acute fatigue in head and neck cancer radiation therapy. Radiother Oncol. 2018;126(1):100–106.
- Rosenthal DI, Mendoza TR, Fuller CD, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer. 2014;120(13):1975–1984.
- Ahn PH, Lukens JN, Teo BK, et al. The use of proton therapy in the treatment of head and neck cancers. Cancer J. 2014;20(6):421–426.
