Abstract
Introduction: To find the optimal dose prescription strategy for liver SBRT, this study investigated the tradeoffs between achievable target dose and healthy liver dose for a range of isotoxic uniform and non-uniform prescription level strategies.
Material and methods: Nine patients received ten liver SBRT courses with intrafraction motion monitoring during treatment. After treatment, five VMAT treatment plans were made for each treatment course. The PTV margin was 5 mm (left-right, anterior-posterior) and 10 mm (cranio-caudal). All plans had a mean CTV dose of 56.25 Gy in three fractions, while the PTV was covered by 50%, 67%, 67 s% (steep dose gradient outside CTV), 80%, and 95% of this dose, respectively. The 50%, 67 s%, 80%, and 95% plans were then renormalized to be isotoxic with the standard 67% plan according to a Lyman-Kutcher-Burman normal tissue complication probability model for radiation induced liver disease. The CTV D98 and mean dose of the iso-toxic plans were calculated both without and with the observed intrafraction motion, using a validated method for motion-including dose reconstruction.
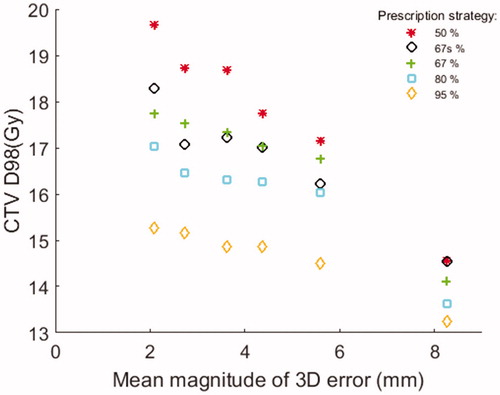
Results: Under isotoxic conditions, the average [range] mean CTV dose per fraction decreased gradually from 21.2 [20.5—22.7] Gy to 15.5 [15.0—16.6] Gy and the D98 dose per fraction decreased from 20.4 [19.7–21.7] Gy to 15.0 [14.5–15.5] Gy, as the prescription level to the PTV rim was increased from 50% to 95%. With inclusion of target motion the mean CTV dose was 20.5 [16.5–22.5] Gy (50% PTV rim dose) and 15.4 [13.9–16.7] Gy (95% rim dose) while D98 was 17.8 [7.4–20.6] Gy (50% rim dose) and 14.6 [8.8–15.7] Gy (95% rim dose).
Conclusion: Requirements of a uniform PTV dose come at the price of excess normal tissue dose. A non-uniform PTV dose allows increased CTV mean dose at the cost of robustness toward intrafraction motion. The increase in planned CTV dose by non-uniform prescription outbalanced the dose deterioration caused by motion.
Introduction
Stereotactic body radiotherapy (SBRT) is a treatment technique where high ablative doses are administered to well defined tumors with high accuracy. During the last decade, SBRT has gained acceptance as an effective treatment modality especially for tumors in the liver and lungs [Citation1–5]. The ratio between tumor dose and adjacent normal tissue dose should be as high as possible in order to maximize the therapeutic ratio of SBRT [Citation6–8], but the actually delivered dose distribution is often degraded due to treatment uncertainties [Citation9–11]. For tumors in the liver, the treatment uncertainties include substantial breathing motion and baseline shifts of the mean tumor position within the patient [Citation9–12]. To mitigate the effects of geometric uncertainties, a safety margin around the clinical target volume (CTV) is added to form the planning target volume (PTV) [Citation13]. In conventionally fractionated radiotherapy, a uniform dose is typically prescribed to the full PTV. However, as pointed out by Craft et al. [Citation14] dose uniformity across the PTV often comes at the price of an increased risk of side effects through excessive normal tissue exposure. Craft et al. urged the radiotherapy community to investigate the tradeoff between target dose uniformity and normal tissue sparing by exploring non-uniform dose prescription strategies with high doses to the CTV and lower doses at the rim of the PTV [Citation14]. Non-uniform prescribed doses have been used in SBRT treatments since the introduction of the technique in the 1990s [Citation15,Citation16]. However, due to a lack of reports investigating the tradeoffs between uniform and non-uniform dose prescription there is presently no consensus on the optimal prescription level strategy in SBRT. Hence, a wide range of dose prescription strategies ranging from 60% to 95% at the PTV rim relative to the prescribed CTV dose are presently used [Citation17,Citation18]. This lack of consensus constitutes a serious problem for comparison and evaluation of clinical SBRT results across different centers [Citation17].
Based on a group of patients with intra-treatment motion monitoring during their liver SBRT treatments [Citation12], this study investigated the tradeoff between achievable target doses and surrounding healthy liver tissue doses for a range of non-uniform dose prescription level strategies. The dose that could be prescribed to the CTV while maintaining the same risk of radiation induced liver disease (RILD) was determined across the different prescription level strategies assuming static patient anatomy. Secondly, the dosimetric consequences of actual intra-treatment motion measured for the included patients were investigated for the various prescription scenarios.
Material and methods
The overall workflow of this study is sketched in . Five volumetric modulated arc therapy (VMAT) plans with the same mean CTV dose, but different dose levels at the PTV rim and therefore different degrees of non-uniformity, were created for nine patients (ten courses) previously treated with liver SBRT [Citation12]. The PTV rim doses ranged from 50% to 95% (. The PTV67 and PTV67s plans defined in both had PTV rim doses of 67%, but the PTV67s plans were optimized to achieve a steeper dose fall-off in the region immediately outside the CTV. This is shown schematically in . Next, each plan was renormalized to isotoxicity with respect to the risk of RILD as determined by a Lyman-Kutcher-Burman (LKB) normal tissue complication probability (NTCP) model [Citation7,Citation19] (). Finally, delivery of the isotoxic plans, which in this paper always refers to the risk of RILD, was simulated with the actual tumor motion measured during treatment delivery [Citation12], and the resulting CTV dose was determined for each simulated fraction with a motion-including dose-reconstruction method [Citation20] (). The following sections describe in detail the methodology of the steps outlined in .
Figure 1. (A) Workflow of the study. (B) Schematic illustration of the dose gradients in the PTV of the different plan types.

Figure 2. Mean and standard deviation (error bars) of the intrafraction tumor position error during each simulated treatment fraction (three fractions per course) in the left-right (LR) cranio-caudal (CC), and anterior-posterior (AP) directions. As indicated, treatment courses 6–10 were performed with abdominal compression.
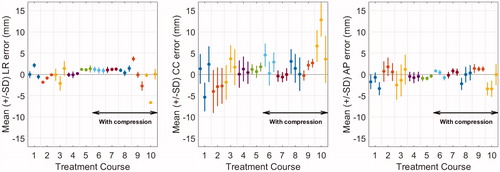
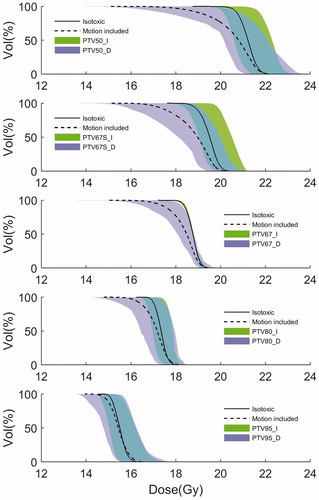
Figure 3. Mean dose volume histograms (DVH) for the CTV for each prescription level strategy across all patients for the isotoxic plans without (_I, solid curve) and with (_D, dashed curve) the dosimetric effects of intrafraction motion. The shaded areas indicate the 10th to 90th percentile range.
Patients and treatment planning
The nine patients were treated with three-fraction SBRT for liver tumors on a Varian Trilogy linear accelerator guided by CBCT setup and simultaneous fluoroscopic MV/kV imaging of implanted fiducial gold markers during treatment as described previously [Citation12,Citation21]. Since one patient received two independent treatment courses, the study includes 10 treatment courses in total. One additional patient of the original cohort [Citation12,Citation21] was excluded because tumor size and proximity to normal tissue hindered production of treatment plans that respected the conditions of the present study. The treatment planning was conducted on the mid-ventilation phase bin of a 10-bin 4DCT scan for nine treatment courses and on a free-breathing 3DCT for one course. A Stereotactic Body Frame (Elekta, Crawley, UK) was used for immobilization. Abdominal compression was applied for five out of the ten treatment courses. For patients with multiple lesions, only the CTV of the lesion closest to the fiducial markers was included in the present study. The mean [range] CTV volume was 37.7 cm3 [0.7—144.3 cm3]. The CTV was located within 5 mm of the liver surface for seven treatment courses and more centrally in the liver for three courses. The PTV was in all cases constructed by adding a margin of 10 mm to the CTV in the cranio-caudal (CC) directions and 5 mm in the left-right (LR) and anterior-posterior (AP) directions.
For each treatment course, five treatment plans were retrospectively created with a 3-field non-coplanar 6 MV VMAT technique (Eclipse 13.7 treatment planning system, AAA dose algorithm, Varian Medical Systems). The gantry angles of the three VMAT arcs were 45°–181° (table 345° and 15°) and 30°–330° (table 90°), and the collimator angle was 45°. The five treatment plans had different strategies for dose fall-off (non-uniformity) outside the CTV. The plans were initially normalized to deliver a mean CTV dose of 56.25 Gy (= 100%) in three fractions (18.75 Gy per fraction) while the dose at the rim of the PTV was 50%, 67 s%, 67%, 80%, and 95% for the plans denoted PTV50, PTV67s, PTV67, PTV80, and PTV95, respectively (). The PTV67 plans represented our clinical standard prescription. The PTV67s plans covered the PTV with 67% dose similar to the PTV67 plans, but higher priority was given to reduce the dose to the region between the CTV and PTV as much as possible while maintaining CTV and PTV coverage (i.e., sharp dose fall-off just outside the CTV). At least 98% of the CTV was covered by the 95% isodose and 98% of the PTV was covered by the prescribed PTV rim dose. The maximum dose (D1cm3) in the CTV was below 107% of the prescription dose. A standard set of optimization constraints was developed to fulfill these requirements while minimizing the dose to the surrounding liver tissue (at least 700 cm3 of liver below 15 Gy). Standard in-house clinical dose-constraints were also applied to other critical normal tissue in order to generate clinically acceptable dose distributions (spinal cord Dmax <18 Gy; esophagus, stomach, intestines, duodenum Dmax(1ccm) <21 Gy, heart Dmax(1ccm) <30 Gy; kidney (bilateral) D15Gy <35% of volume).
Renormalization to isotoxicity
For each of the five treatment plans in a course, the NTCP for RILD was calculated from the dose distribution to the liver (excluding the CTV) by a clinically used LKB-NTCP model developed by Dawson et al. for patients with liver metastases [Citation7,Citation19]. The model parameters applied were TD50 = 45.8 Gy, m = 0.12 and n = 0.97. Originally, this model was derived from patients treated with 1.5 Gy per fraction. To correct for the large difference in fractionation, the dose volume histograms (DVHs) were scaled to 1.5 Gy per fraction before calculation of the NTCP, using the linear quadratic model with an α/β ratio of 2.5 Gy as previously demonstrated for liver SBRT use [Citation19]. Note that the aim of the NTCP estimation was not to determine absolute RILD risks, but to allow renormalization of the plans to the same toxicity risk.
Based on the NTCP model, isotoxic plans (PTV50_I, PTV67s_I, PTV67_I, PTV80_I, PTV95_I) with the same risk of RILD as the PTV67 plan were created by renormalization (). Note that the PTV67_I and PTV67 plans were identical. The renormalized isotoxic plans had the same relative dose distribution as the original plans, but different absolute CTV doses. No normal tissue constraint was violated as a result of the renormalization.
Treatment simulations with motion-including dose reconstruction
Treatment delivery of the five isotoxic plans including the consequences of intrafraction motion was simulated for the three fractions of each treatment course (). This simulated delivery was based on the time-resolved 3D tumor motion previously measured during the actual clinical treatment delivery by fluoroscopic x-ray imaging as reported in [Citation12] where detailed examples of the intrafraction motion can be found. The simulations assumed that the patients were accurately set up to the mean internal marker position during the setup CBCT acquisition [Citation22]. Hence, the intrafraction motion in the treatment simulations included respiratory motion during treatment and possible baseline shifts between setup CBCT and field delivery. The treatment simulations assumed VMAT delivery according to the treatment plan (i.e., no machine errors) with the gantry speed limitation (4.8°/s) and dose rate limitation (92% of the nominal setting of 600 MU/min) measured for a Trilogy accelerator. The simulations resulted in all treatment machine parameters as function of time, synchronized with the tumor motion as function of time. This information was used for motion-including dose reconstruction by an experimentally validated method that models the translational motion of a rigid target as multiple isocenter shifts by manipulating the original treatment plan in an in-house software tool. The manipulated plan with isocenter shifts corresponding to the observed motion was imported back into the treatment planning system to calculate the delivered target dose including the effect of motion [Citation10,Citation20]. A 1.5 mm bin width was used, i.e., all target positions within a 1.5 x 1.5 x 1.5mm3 cube were given the same isocenter shift. Each plan resulted in three simulated delivered dose distributions with the motion measured at the three treatment fractions per course (denoted with subscript ‘D’ in ).
Data analysis
The conformity index of the initial plans () was calculated as the total volume receiving the prescribed relative PTV rim dose (i.e., 95%, 80%, 67%, 50% isodoses) divided by the PTV volume. The intra-fraction tumor position error during each simulated treatment fraction was quantified as the mean error (i.e., systematic error) and the standard deviation of the error (i.e., random error). The mean dose to the CTV and the minimum dose to 98% of the CTV (D98) were extracted (1) for each isotoxic plan () to represent the planned CTV dose under static conditions, and (2) for each simulated treatment fraction () to represent the delivered doses including motion effects. For each prescription level strategy, mean DVHs (with 10th and 90th percentiles) were generated for the patient population by calculating the mean and percentiles across the individual DVHs for each volume level. Statistical comparison of CTV doses between the different prescription level strategies was performed using Wilcoxon signed-rank test.
Results
NTCP values, conformity index and tumor position errors
The median [range] NTCP for RILD of the isotoxic plans for the 10 SBRT courses was 0.1% [0.0–39.2%]. In all cases except one all other normal tissue constraints were met. In a single case the proximity of the tumor to the heart resulted in 20–52 cm3 of heart-tissue receiving above 30 Gy for the plans generated for this study. The mean (± SD) conformity index was 1.91 ± 0.27 (PTV50 plans), 1.44 ± 0.20 (PTV67s plans), 1.24 ± 0.04 (PTV67 plans), 1.12 ± 0.03 (PTV80 plans), and 1.05 ± 0.01 (PTV95 plans). The intrafraction systematic and random tumor position errors varied substantially between treatment fractions (). The mean absolute systematic geometrical errors during the simulated treatment fractions were 1.2 [0.0–6.6] mm (LR), 2.4 [0.0–12.8] mm (CC) and 1.2 [0.0–3.4] mm (AP), and the random errors were 0.7 [0.2–1.8] mm (LR), 2.9 [1.0–5.5] mm (CC) and 1.4 [0.4–3.7] mm (AP). The systematic errors were caused by baseline shifts of the mean tumor position between setup CBCT and treatment delivery and the random errors were caused by respiratory motion and intra-treatment baseline shifts.
Isotoxic planning strategies
The use of more non-uniform prescription strategies resulted in increasingly higher planned static CTV doses as compared to the uniform PTV95_I plans (). While the PTV67_I plans had very similar CTV DVHs (narrow standard deviation band in ), the other plans had larger DVH variations caused by the renormalization to be isotoxic with the PTV67_I plans. The intrafraction motion in general shifted the CTV DVHs to lower dose levels (). This motion-induced CTV dose degradation was clearly more prominent for the more non-uniform prescription level strategies, indicating less robustness against intrafraction motion. Still, the mean DVHs remained highest for the more non-uniform prescription level strategies even when motion was included ().
summarizes the CTV Dmean and D98 of all prescription level strategies both without and with intrafraction motion. Since the static isotoxic plans were normalized according to CTV mean dose, the ratios between the Dmean static doses in (top row) and the Dmean (18.8 Gy) of the standard 67% strategy corresponds to the scaling factors applied to achieve isotoxic plans with respect to the risk of RILD. For the static isotoxic plans, the PTV50_I plans on average had CTV doses that were 36.8% (Dmean) and 36.0% (D98) higher than the PTV95_I plans. Intrafraction motion on average reduced the CTV dose by 0.7 Gy (3.3%) (Dmean) and 2.7 Gy (12.7%) (D98) for the PTV50_I plans and by 0.1 Gy (0.6%) (Dmean) and 0.4 Gy (2.7%) (D98) for the PTV95_I plans. With motion the mean D98 remained 21.9% higher for the PTV50_D doses than for the PTV95_D doses. The D98 was higher with the PTV50_D doses (and PTV67_D doses) than with the PTV95_D doses in 28 (and 29) out of the 30 simulated fractions, i.e., for the two fractions with largest intrafraction motion, the delivered CTV D98 was lower with the non-uniform 50% prescription strategy than with the uniform 95% strategy. The CTV D98 and Dmean of the motion including simulated treatments differed significantly (p < .01) between all prescription level strategies, except for D98 between the PTV67_D and PTV67s_D plans (p = .68).
Table 1. Average [range] CTV mean fraction dose (Dmean) and minimum dose to 98% of the CTV (D98) for the isotoxic plans as planned (static) and with simulated delivery including tumor motion.
The delivered CTV dose in general decreased with increasing geometrical errors (). This trend was more pronounced for the less uniform dose prescription strategies, especially at fractions with large intrafraction motion comparable to the size of the PTV margin. On average, the mean liver dose per fraction for the different isotoxic static plan strategies were 3.2 [1.3–4.9] Gy (PTV50_I), 3.2 [1.3–5.0] Gy (PTV67s_I), 3.2 [1.3–5.1] Gy (PTV67_I), 3.3 [1.3–4.9] Gy (PTV80_I), and 3.3 [1.3–5.0] Gy (PTV95_I), respectively. For all strategies, inclusion of motion on average resulted in a change in mean liver dose of 0.0 [−0.2 – 0.2] Gy.
Discussion
This study investigated different PTV prescription level strategies for liver SBRT with respect to the achievable target dose under isotoxic conditions as modeled by the risk of RILD. Non-uniform prescribed PTV doses with lower dose at the PTV rim than in the CTV allowed isotoxic boosting of the planned mean CTV dose by up to 36.8% in mean, compared to a uniform prescription strategy (, 50% versus 95% strategy). Hence, requiring a high dose in the PTV region outside the CTV had a large effect on the risk of normal tissue complication. However, the tumor may move outside the high dose volume during treatment delivery. Treatment simulations with the actual intra-treatment tumor motion showed that non-uniform plans with dose fall-off outside the CTV were clearly less robust toward intrafraction motion than uniform plans ( and ). Still, under isotoxic conditions the non-uniform prescription level strategies provided higher mean CTV dose than the uniform 95% strategy even when motion was included.
The idea of non-uniform dose prescriptions was introduced together with SBRT in the mid-1990s where Swedish studies indicated that it was possible to boost the dose in the central CTV region almost without increasing the normal tissue dose [Citation15,Citation16]. In a more recent liver SBRT study, de Pooter et al. compared treatment planning based on PTV prescription isodoses of 65% and 80%, respectively, relative to the isocenter dose [Citation23]. In this study treatment plans were not normalized for isotoxicity but optimized to obey a set of strict constraints to the normal tissues while trying to maximize the central isocenter dose. Also, intrafraction motion was not explicitly modeled. However, Pooter and coworkers concluded that the non-uniform 65% dose prescription level strategy did provide isocenter and mean PTV doses that were 17.6% and 9.8% higher, respectively, than the more uniform 80% strategy while maintaining approximately the same dose to the surrounding normal tissue. For lung SBRT, Guckenberger et al. investigated the use of 65% and 80% PTV prescription strategies based on dose accumulation in 4DCT scans [Citation24]. They concluded that treatment planning based on the 65% prescription strategy increased the isocenter dose with 23% with no significant difference in the dose to the ipsilateral lung compared to the 80% strategy. Fleckenstein et al. similarly found that compared to a uniform prescription strategy, non-uniform dose prescription reduced the ipsilateral lung dose and increased the target dose in lung SBRT [Citation25]. In another lung SBRT study based on conformal arc techniques, Widder et al. [Citation26] observed that PTV prescription isodoses lower than 80% provided higher dose gradients outside the PTV and therefore better normal tissue sparing than uniform planning strategies. Though these previous studies did not apply rigorous isotoxic planning strategies and did not model intrafraction motion observed during actual treatment, the findings were all in line with the present study where the mean dose to the CTV was 8.7% (without motion) or 7.6% (with motion) higher for the 67% prescription strategy than for the 80% strategy (). Also, compared to the 67% prescription strategy, intentional dose shaping by the 67 s% scenario to achieve a steep dose gradient in the PTV region just outside the CTV further increased the delivered mean CTV dose by 2.2% (). Aiming for prescription strategies below 50% was not feasible while maintaining plan quality, i.e., the 50% prescription strategy represented the maximum achievable dose-fall off from CTV to PTV. In this study the inclusion of intrafraction motion had almost no effect on the mean liver dose.
The present study was based on a biological model for the risk of RILD that was originally derived from data with fraction doses of 1.5 Gy [Citation7]. Although extrapolation to the stereotactic regimen introduces additional uncertainties in the NTCP estimations [Citation19] the RILD model has been applied and found safe in several clinical studies of individualized risk adapted liver SBRT [Citation19,Citation27,Citation28]. The patient with the highest modeled NTCP (39.2%) was treated with a reduced dose of 45 Gy at the actual treatment instead of the 56.25 Gy applied in this simulation study.
In this study, we used PTV margins of 10 mm (CC) and 5 mm (LR,AP), which are commonly used for SBRT in Scandinavia [Citation15,Citation16]. Beside intrafraction motion, there are other uncertainties not modeled in the current study. This includes liver deformations, liver rotations, tumor motion relative to the fiducial markers, and errors in the online match between the setup CBCT and the planning CT [Citation29–31]. Consequently, our motion including treatment simulation overestimated the dose that would be delivered to the CTV if all sources of error were included. Despite this limitation the present study clearly demonstrated what was very recently described as the price of uniformity by Craft et al. [Citation14]: Aiming for a uniform dose throughout the full PTV volume does not provide higher delivered CTV doses in liver SBRT under isotoxic conditions.
Based on this study, the 67% prescription strategy applied in Scandinavia delivers a reasonable compromise between achievable target dose and robustness toward intrafraction motion. Future work should seek to quantify and include all sources of geometric errors to fully elucidate the complex interplay between the planned 3D dose-distribution, geometrical errors and achievable dose to the CTV in order to define the optimal dose prescription strategy in liver SBRT. Extension of the study to include other prescription strategies, e.g., peaked dose distributions without the formal 107% dose maximum constraint centrally in the CTV, is also an appealing option that needs investigation [Citation32,Citation33]. Ultimately, the biological consequences of different dose prescription strategies should be investigated in randomized clinical trials to establish the ideal way of SBRT delivery. The ongoing NARLAL2 trial of FDG-guided heterogeneous dose escalation in fractionated radiotherapy of lung tumors is an example of randomization between prescription strategies [Citation32,Citation33]. Also, the present study used the risk of RILD alone to benchmark the burden to the normal tissue. The dose to other organs at risk could be included in further analyses. However, other organs are often not located near the target and are typical of serial nature with an achievable near maximum dose constraint and are therefore not suitable for general benchmarking and isotoxicity normalization.
In conclusion, dose uniformity throughout the PTV causes excess dose to the normal tissue and limits the achievable dose to the CTV. By prescribing a non-uniform dose the CTV dose may be increased at the cost of robustness toward intrafraction motion. However, the potential increase in planned CTV dose by non-uniform prescription strategies clearly outbalanced the dose deterioration caused by intrafraction motion in this study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Høyer M, Muren LP. Stereotactic body radiation therapy – a discipline with Nordic origin and profile. Acta Oncol. 2012;51(5):564–567.
- Fode MM, Hoyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol. 2015;114(2):155–160.
- Lock MI, Hoyer M, Bydder SA, et al. An international survey on liver metastases radiotherapy. Acta Oncol. 2012;51(5):568–574.
- Guckenberger M, Klement RJ, Allgauer M, et al. Local tumor control probability modeling of primary and secondary lung tumors in stereotactic body radiotherapy. Radiother Oncol. 2016;118(3):485–491.
- Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol. 2013;106(3):276–282.
- Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases. Cancer. 2011;117(17):4060–4069.
- Dawson L, Tenhaken R. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15(4):279–283.
- Van den Begin R, Engels B, Gevaert T, et al. Impact of inadequate respiratory motion management in SBRT for oligometastatic colorectal cancer. Radiother Oncol. 2014;113(2):235–239.
- Poulsen PR, Worm ES, Hansen R, et al. Respiratory gating based on internal electromagnetic motion monitoring during stereotactic liver radiation therapy: first results. Acta Oncol. 2015;54(9):1445–1452.
- Poulsen PR, Worm ES, Petersen JB, et al. Kilovoltage intrafraction motion monitoring and target dose reconstruction for stereotactic volumetric modulated arc therapy of tumors in the liver. Radiother Oncol. 2014;111(3):424–430.
- Worm ES, Hoyer M, Hansen R, et al. A prospective cohort study of gated stereotactic liver radiation therapy using continuous internal electromagnetic motion monitoring. Int J Radiat Oncol Biol Phys. 2018;101(2):366–375.
- Worm ES, Høyer M, Fledelius W, et al. Three-dimensional, time-resolved, intrafraction motion monitoring throughout stereotactic liver radiation therapy on a conventional linear accelerator. Int J Radiat Oncol Biol Phys. 2013;86(1):190–197.
- International Commision on Radiation Units and Measurements. ICRU report 62 (supplement to ICRU Report 50). Prescribing, recording, and reporting photon beam therapy. Bethesda, MD: ICRU; 1999.
- Craft D, Khan F, Young M, et al. The price of target dose uniformity. Int J Radiat Oncol Biol Phys. 2016;96(4):913–914.
- Lax I, Blomgren H, Naslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33(6):677–683.
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator: clinical experience of the first thirty-one patients. Acta Oncol. 1995;34(6):861–870.
- Guckenberger M. Dose and fractionation in stereotactic body radiation therapy for stage i non-small cell lung cancer: lessons learned and where do we go next? Int J Radiat Oncol Biol Phys. 2015;93(4):765–768.
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101.
- Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006;45(7):856–864.
- Poulsen PR, Schmidt ML, Keall P, et al. A method of dose reconstruction for moving targets compatible with dynamic treatments. Med Phys. 2012;39(10):6237–6246.
- Worm ES, Hoyer M, Fledelius W, et al. Variations in magnitude and directionality of respiratory target motion throughout full treatment courses of stereotactic body radiotherapy for tumors in the liver. Acta Oncol. 2013;52(7):1437–1444.
- Worm ES, Høyer M, Fledelius W, et al. On-line use of three-dimensional marker trajectory estimation from cone-beam computed tomography projections for precise setup in radiotherapy for targets with respiratory motion. Int J Radiat Oncol Biol Phys. 2012;83(1):e145–e151.
- de Pooter JA, Wunderink W, Mendez Romero A, et al. PTV dose prescription strategies for SBRT of metastatic liver tumours. Radiother Oncol. 2007;85(2):260–266.
- Guckenberger M, Wilbert J, Krieger T, et al. Four-dimensional treatment planning for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(1):276–285.
- Fleckenstein J, Boda-Heggemann J, Siebenlist K, et al. Non-coplanar VMAT combined with non-uniform dose prescription markedly reduces lung dose in breath-hold lung SBRT. Strahlenther Onkol. 2018;194(9):815–823.
- Widder J, Hollander M, Ubbels JF, et al. Optimizing dose prescription in stereotactic body radiotherapy for lung tumours using Monte Carlo dose calculation. Radiother Oncol. 2010;94(1):42–46.
- Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27(10):1585–1591.
- Aitken KL, Tait DM, Nutting CM, et al. Risk-adapted strategy partial liver irradiation for the treatment of large volume metastatic liver disease. Acta Oncol. 2014;53(5):702–706.
- Bertholet J, Worm ES, Fledelius W, et al. Time-resolved intrafraction target translations and rotations during stereotactic liver radiation therapy: implications for marker-based localization accuracy. Int J Radiat Oncol Biol Phys. 2016;95(2):802–809.
- Worm ES, Bertholet J, Hoyer M, et al. Fiducial marker guided stereotactic liver radiotherapy: is a time delay between marker implantation and planning CT needed? Radiother Oncol. 2016;121(1):75–78.
- Bertholet J, Worm E, Hoyer M, et al. Cone beam CT-based set-up strategies with and without rotational correction for stereotactic body radiation therapy in the liver. Acta Oncol. 2017;56(6):860–866.
- Møller DS, Nielsen TB, Brink C, et al. Heterogeneous FDG-guided dose-escalation for locally advanced NSCLC (the NARLAL2 trial): design and early dosimetric results of a randomized, multi-centre phase-III study. Radiother Oncol. 2017;124(2):311–317.
- Nielsen TB, Hansen O, Schytte T, et al. Inhomogeneous dose escalation increases expected local control for NSCLC patients with lymph node involvement without increased mean lung dose. Acta Oncol. 2014;53(1):119–125.