Abstract
Objectives: This real-world study on small-cell lung cancer (SCLC) patients aimed to investigate treatment patterns, outcome of re-challenge with platinum doublet chemotherapy (PDCT), and associations between clinical characteristics and survival outcomes.
Material and methods: This retrospective single center cohort study was based on patients diagnosed with SCLC between 2008 and 2016 at the Karolinska University Hospital, Stockholm, Sweden. Patients were divided into two subgroups; limited disease (LD), receiving concomitant chemo- and radiotherapy and extensive disease (ED), receiving palliative PDCT. The progression-free survival (PFS) was defined as the interval between the start of CT and the earliest date of documented progression. ‘Refractory relapse’ (Rr) and ‘Sensitive relapse’ (Sr) were defined as relapse occurring < or ≥180 days after start of PDCT, respectively. The results for treatment patterns were reported as numbers and percentages of patients, and descriptive analyses including medians and 95% confidence intervals (CIs). The Cox proportional hazards regression model was applied to assess the relationship between clinical characteristics and overall survival (OS).
Results: The study included 544 patients; 408 with ED and 136 patients had LD. The median PFS and OS for ED patients were 5.1 and 7.0, respectively. In the ED subgroup, Sr occurred in 169 patients (41%), with a longer median OS when compared to Rr patients (10.8 vs. 3.6 months). Patients with LD had a median PFS and OS of 12 and 24 months, respectively. Some LD patients did not show a sign of relapse (22%). The majority of LD patients who relapsed had Sr (66%), with a longer median OS when compared to patients with Rr (20.9 vs. 7.8 mo).
Conclusions: The survival outcomes for ED and LD SCLC patients correspond to historical data. Patients with Sr after 1st line therapy might benefit from re-challenge with PDCT in the 2nd line setting.
Background
The current standard of care for limited disease (LD) small-cell lung cancer (SCLC) patients is platinum doublet chemotherapy (PDCT) administered for four to six cycles with concomitant thoracic radiotherapy (CT + RT). The most frequently used schedule for concomitant radiation therapy consists of 1.5 Gy fractions, twice per day, up to a total dose of 45 Gy [Citation1]. For extensive disease (ED), the standard of care for 1st line treatment consists of the similar PDCT as in LD but without the addition of concomitant RT. The treatment options for ED have recently been enriched by the introduction of atezolizumab, an anti-programed death-ligand 1 (PD-L1) inhibitor, which can be used together with PDCT in patients with excellent performance status (PS) [Citation2]. The prognosis for SCLC patients receiving only best supportive care (BSC) is 2–4 months [Citation1]. Despite high initial treatment responses to PDCT (70%), almost all patients inevitably develop resistance and relapse [Citation3]. Patients receiving PDCT who have signs of relapse can be divided into refractory and sensitive groups. The definitions of platinum ‘sensitive’ and ‘refractory’ disease vary between previous studies; either with progression-free survival (PFS) of ≥ or <180 days from the start of 1st line treatment, or of ≥ or <90 days after the end of PDCT, respectively [Citation3–5]. Patients without signs of relapse after 1st line therapy are not included in these definitions. The practical value of such definitions is to correlate platinum sensitivity with prognosis and suggest secondary treatment strategies. Hence, patients with ‘sensitive relapse’ (Sr) are recommended re-challenge with PDCT, while patients with ‘refractory relapse’ (Rr) are recommended a non-platinum based regimen as second line (2nd line). These recommendations though, are not based on results from randomized clinical trials [Citation5]. Topotecan, in fact, is the only FDA-approved agent for 2nd line treatment in SCLC, based on the results of three phase III trials [Citation6–8]. These trials compared topotecan with anthracycline-based CT or BSC [Citation6–9]. Monotherapy with nivolumab is the only FDA-approved drug for 3rd line treatment, based on an open-labeled phase I/II study with a PFS rate of 17.2% at six months [Citation10]. In addition, few SCLC patients are still in adequate physical condition to receive 3rd line treatments [Citation9]. For LD patients who respond to CT + RT, prophylactic cranial irradiation (PCI) both increases overall survival (OS) and decreases the incidence of brain metastases [Citation11]. However, the clinical benefit of PCI in ED patients remains uncertain, since results from two randomized clinical trials have shown conflicting results [Citation12,Citation13]. The National Swedish guidelines recommend using PCI for all LD patients with response to 1st line treatment and for ED patients without signs of rapid progression after 1st line CT and with a good PS. Furthermore, patients aged over 80 years or with cerebrovascular disease are not recommended PCI [Citation14,Citation15].
Given this background, the aim of the present large retrospective study was to evaluate the treatment patterns for SCLC patients in 1st, 2nd and 3rd line as well as the outcomes of re-challenge with PDCT compared to previous experiences [Citation16,Citation17]. The purpose was also to investigate if there was an association between clinical characteristics and outcomes in SCLC patients in a real-world setting.
Material and methods
Data collection
This was a non-interventional, retrospective, single-center cohort study. The study included patients with a first-time diagnosis of SCLC who had completed ≥1 cycle of PDCT with or without concomitant RT between 1 January 2008 and 1 February 2016. All patients included in the study were identified from the Swedish Lung Cancer Registry and had been treated at the Karolinska University Hospital, Stockholm, Sweden. All data regarding clinical characteristics, treatment patterns and survival outcomes were manually retrieved from each patient's medical record by one of the study authors (ST). Tumor stage was reclassified according to the 8th TNM system for all patients [Citation18]. However, for the present analyses, cases were categorized according to the Veterans Administration Lung Study Group (VASLG) classification system (LD/ED) based on the treatment intention [Citation19]. The last follow-up date was 30 June 2018.
Five hundred and sixty patients diagnosed with SCLC and treated at the Karolinska University Hospital during the study period were identified in the Swedish Lung Cancer Registry. Patients with LD who interrupted their concomitant RT due to toxicity or progression (n = 4), as well as patients with ED who were treated with concomitant CT + RT in the 1st line were excluded (n = 12). The final study cohort encompassed 544 SCLC patients.
Treatment patterns
Subjects were divided into two groups depending on the intention of 1st line treatment; palliative chemotherapy for ED patients and curative intended CT + RT for LD patients. The RT scheduling followed local clinical guidelines; 45 Gy, delivered in 30 fractions, twice daily, starting with the second course of PDCT [Citation15].
The most commonly used PDCT regimen was Carboplatin, together with etoposide or irinotecan (EP/IP). The CT agents used in monotherapy in 2nd line included topotecan (T), etoposide (E) and irinotecan (I). Patients received PCI according to local guidelines [Citation14,Citation15].
Co-variates
The following clinical characteristics were included: PS, age, gender, smoking status and TNM stage at the time of diagnosis. Smoking status was divided into current, previous or nonsmoker, and was based on self-reporting from the patients. The PS was retrieved prior to initiating 1st line treatment. The following hematology and blood chemistry values were obtained prior to 1st line treatment: hemoglobin, C-reactive protein (CRP), lactate dehydrogenase, albumin (Alb) and sodium (Na). The distribution of Hb (g/L) was relatively large, with an interquartile range (IQR) of 100–180 g/L, and therefore, in the uni- and multi-variate analyses, an increase with 20 g/L was chosen. A log-transformed approach was instead chosen for CRP and LDH since the distribution of these lab values was skewed. These clinical and laboratory characteristics were chosen since they are routinely obtained at Karolinska University Hospital and previously have been shown to be prognostically relevant in SCLC patients [Citation20,Citation21].
Definitions of outcomes
The index date was defined as the starting date of CT. The PFS for 1st line therapy was the interval between the index date and the earliest date of documented clinical or radiological progression according to standard clinical practice, or death. The PFS for 2nd and 3rd line therapy was defined according to the index date of each line of therapy until documented clinical or radiological progression according to standard clinical practice, or death. Rr and Sr were defined as relapse occurring within or later than 180 days from the start of PDCT, respectively. We chose this cutoff, rather than 90 days after completion of treatment to minimize potential bias originating from differences in CT schedules, with etoposide administered for three to 14 days, and in the total number of CT cycles due to toxicity or patient choice [Citation1]. Patients who did not have verified relapse were not included in the Sr subgroup, but were instead classified as ‘no relapse’. Re-challenge with the same CT agents after completion of a planned course was considered as a subsequent line of treatment. OS was defined as the interval between the index date of each line of CT until death. Patients who did not receive subsequent treatment despite tumor progression were defined as BSC.
Statistical analysis
The data on baseline characteristics and treatment patterns were summarized using frequency counts with percentages and medians with 95% confidence intervals (CIs) [Citation22].
All analyses were separately conducted for patients with ED and LD since these subgroups have different survival outcomes [Citation23]. The median PFS and OS were estimated in months, with a p value of <.05 considered statistically significant.
The Cox proportional hazards regression was used to assess the relationship between clinical parameters prior to starting 1st line therapy and OS. In multivariable analyses, all baseline characteristics were included. All statistical analyses were conducted using R 3.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
The number of patients included in the study was 544, with 219 (40%) being males. Four hundred and eight patients were diagnosed with ED while 136 patients had LD. The median age at diagnosis was 70.5 years and 67.2 years for ED and LD patients, respectively. The number of patients with PS 0–1 was 380 (70%), while 158 subjects had PS 3–4 (29%). Baseline characteristics for the ED and, separately, for the LD subgroup are presented in . Only eight patients were never-smokers. A larger proportion of LD patients had PS 0 (56%) when compared to the ED group (23%). Some patients (n = 48) with stage III were defined as ED, because the tumor burden was too large to be included in a single radiation field. A small number of patients in the LD group (n = 8) received 50 Gy, delivered in 25 fractions or 60 Gy delivered in 30 fractions, once per day. The number of patients with LD and ED SCLC receiving PCI was 116 and 74, respectively.
Table 1. Baseline characteristics for extensive disease (ED) and limited disease (LD) patients.
Treatment patterns and outcomes for ED patients
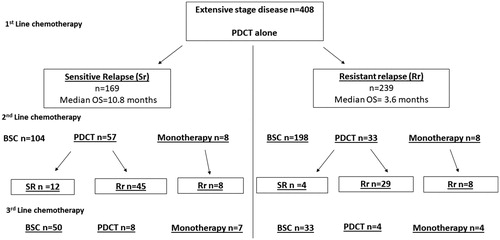
The median PFS and OS among ED patients receiving 1st line PDCT were 5.1 and 7.0 months, respectively. One-fourth of the patients received a 2nd line treatment, while the rest received BSC only. A small proportion (5%) of patients carried on receiving 3rd line treatment, with a poor prognosis independently of whether they were administered PDCT or monotherapy ().
Table 2. Treatment patterns and survival outcomes for each line of chemotherapy for extensive disease (ED) and limited disease (LD) patients.
Patients who were not administered PCI, had a PS ≥3, elevated LDH values and stage IV disease had a statistically significantly worse prognosis in the multivariable analysis ().
Table 3. Uni- and multi-variate survival analyses for extensive disease (ED) and limited disease (LD) patients.
Re-challenge with PDCT for ED patients
The proportion of ED patients sensitive to PDCT in the 1st line was 41%. The median OS from the start of 1st line for Sr was 10.8 months and 3.6 months for the Rr subgroup. A clear majority of patients with Rr in the 1st line received BSC only (83%). Re-challenge with PDCT was the most common treatment option in the 2nd line for both Sr and Rr subgroups. Median OS after start of 2nd line therapy in the Sr and Rr groups was 10.2 and 4.4 months, respectively. In the Rr subgroup, most patients continued having Rr (88%), with a short median OS (3.6 months), presented in .
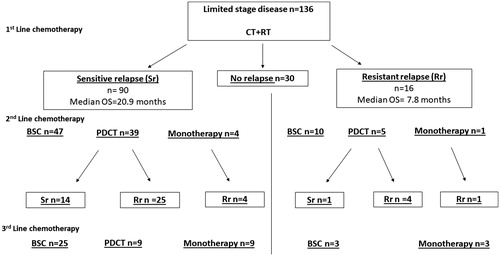
Treatment patterns and outcomes for LD patients
Patients with LD receiving CT + RT in 1st line had a median PFS and OS of 12.3 months and 24.2 months, respectively. Some patients in the LD subgroup did not show signs of relapse at the end of the study (22%). More than one-third of the patients received 2nd line treatment, with a majority being re-challenged with PDCT. The overall median PFS in the 2nd line was 4.8 months, while the median OS was 8.2 months. Few patients received monotherapy in the 2nd line, and they generally had a poor prognosis. A larger proportion of patients received 3rd line therapy when compared to the ED group, with similar median PFS/OS to the ED group ().
In the multivariable analyses, none of the clinical factors examined were statistically significant prognostic factors for OS ().
Re-challenge with PDCT for LD patients
Most LD patients had Sr in the 1st line treatment (66%) with a median OS of 20.9 months, while Rr patients had median OS of 7.8 months. In the 2nd line, most patients were re-challenged with PDCT, and 36% of them continued showing Sr. The Sr patients had a median OS of 15.6 months, while Rr patients had a median OS of 6.6 months. In the Rr subgroup, few patients received 2nd and 3rd line treatment ().
Discussion
The purpose of the present Swedish single center cohort study was to analyze real-world data on SCLC treatment patterns in the 1st, 2nd and 3rd line of therapy, as well as outcomes of re-challenge with PDCT. We wanted to investigate how patients at the Karolinska University Hospital have been treated and compare survival outcomes with previous studies [Citation16,Citation17].
Our results showed that most patients had ED at diagnosis, with a median PFS and OS of 5.1 and 7.0 months, respectively. These results are similar to historical data, with a reported median OS for ED patients of 7–10 months [Citation1,Citation24,Citation25].
The median PFS for both the 2nd and 3rd line CT in the ED subgroup was less than five months, illustrating the difficulty of treating these patients beyond 1st line CT [Citation16]. In addition, ED patients had one to two months interval between disease progression and time of death for each line of CT, emphasizing a very poor prognosis when tumor progression occurs, which is in line with a previous report [Citation24].
Both ED and LD patients receiving PDCT in 2nd line had a longer median PFS and OS when compared to patients treated with monotherapy, which was expected [Citation24]. A small number of patients with LD or ED received 3rd line CT, indicating that randomized clinical trials in this setting are difficult to conduct [Citation16]. From our cohort, it is difficult to make definite conclusions regarding the efficacy of each CT used in the monotherapy setting because of small sample sizes. However, it seems that no CT agent was superior in the monotherapy groups for 2nd and 3rd lines in both LD and ED patients.
A re-challenge with PDCT has been commonly used in Sr patients, based on evidence drawn from small trials [Citation16, Citation24]. In our study, 41% of the ED patients had a Sr after 1st line PDCT, which indicates that many patients developed platinum-resistance early in the course of their disease [Citation3]. In the re-challenged patient subgroup, there was a six months longer median OS when compared to Rr patients. For the Rr subgroup, most re-challenged subjects continued having Rr (88%). This shows that ED patients should be re-challenged based on PFS after 1st line CT [Citation4, Citation26].
Our results point toward the importance of an adequate assessment of PS when evaluating treatment options for patients with ED, as a patients with poor PS may not derive meaningful benefit of PDCT [Citation25]. In our analyses, a high LDH level was an independent prognostic factor in ED patients [Citation21, Citation27]. PCI was also associated with a better prognosis in ED patients as reported before [Citation28]. This is expected since only patients without rapid progression and good PS are candidates for receiving PCI in the ED subgroup.
LD patients were treated with concurrent CT + RT, with the same radiation therapy schedule implemented throughout the entire study period, except for very few cases. A minority of LD patients did not show signs of relapse (22%), which is in accordance with historical data [Citation29,Citation30]. The survival for LD patients receiving CT + RT in the 1st line CT was longer when compared to certain historical data, while shorter when compared to the Convert trial [Citation29, Citation31]. Increased monitoring can be crucial in SCLC, and thus may provide better outcomes in a randomized controlled trial when compared to the real-world setting. The median PFS and OS for LD patients in the 2nd and 3rd line CT settings were similar to what observed in ED, which further illustrates the difficulty to treat patients who have progressed, regardless of the stage of the disease. In the LD subgroup, the median OS for Sr was 20.9 months, when compared to 7.8 months for Rr. Most subjects with Sr were re-challenged with PDCT, and a larger proportion had Sr when compared with ED patients (36% vs. 26%, respectively). The Sr subgroup had a nine months longer median OS when compared to Rr. This illustrates that the PFS to 1st line PDCT is a predictor for survival outcomes for 2nd line CT in LD patients [Citation32]. One possible explanation could be that a longer period without CT might result in a better physical condition when relapses occur, enabling the patient to tolerate subsequent treatments better, leading to longer survival. Another reason could be that the tumor has not yet developed platinum-resistance.
Our study has some limitations. First, data on tumor response to treatment were not collected as different radiological modalities were used to estimate response, and strict evaluation criteria were not consistently implemented, introducing a considerable uncertainty on the accuracy of response assessment. Information on adverse events was not obtained since the retrospective nature of the study made such data difficult to collect. The PS was based on the physician's assessment while smoking history was from self-reporting, which could be subject to recall bias [Citation33]. Finally, the results are not generalized to the subgroup of patients who only receive BSC after being diagnosed with SCLC.
Conclusions
This is to our knowledge one of the largest real-world data analyses to evaluate survival outcomes for 1st, 2nd and 3rd line chemotherapy as well as re-challenge treatment in SCLC. The survival outcomes for LD and ED SCLC patients in the present study correspond to historical data.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Rossi A, Martelli O, Di Maio M. Treatment of patients with small-cell lung cancer: from meta-analyses to clinical practice. Cancer Treatment Rev. 2013;39(5):498–506.
- Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229.
- Naito Y, Yamada K, Imamura Y, et al. Rechallenge treatment with a platinum-based regimen in patients with sensitive relapsed small-cell lung cancer. Med Oncol. 2018;35(5):61.
- Shiozawa T, Sekine I, Aida Y, et al. Rechallenge with first-line platinum chemotherapy for sensitive-relapsed small-cell lung cancer. Case Rep Oncol. 2018;11(3):622–632.
- Genestreti G, Tiseo M, Kenmotsu H, et al. Outcomes of platinum-sensitive small-cell lung cancer patients treated with platinum/etoposide rechallenge: a multi-institutional retrospective analysis. Clin Lung Cancer. 2015;16(6):e223–e228.
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17(2):658–667.
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24(34):5441–5447.
- Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25(15):2086–2092.
- Oronsky B, Reid TR, Oronsky A, et al. What's new in SCLC? A review. Neoplasia (New York, NY). 2017;19(10):842–847.
- Ready N, Farago AF, de Braud F, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14(2):237–244.
- Aupérin A, Arriagada R, Pignon J-P, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341(7):476–484.
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–672.
- Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(5):663–671.
- National Guidelines Sweden, Small Cell Lung Cancer; 2018. [cited 2020 Jan 2]. Available from: https://www.cancercentrum.se/samverkan/cancerdiagnoser/lunga-och-lungsack/vardprogram/
- NGS SCLC. [cited 2020 Jan 2]. Available from: https://www.cancercentrum.se/samverkan/cancerdiagnoser/lunga-och-lungsack/vardprogram/
- Coutinho AD, Shah M, Lunacsek OE, et al. Real-world treatment patterns and outcomes of patients with small cell lung cancer progressing after 2 lines of therapy. Lung Cancer. 2019;127:53–58.
- Steffens CC, Elender C, Hutzschenreuter U, et al. Treatment and outcome of 432 patients with extensive-stage small cell lung cancer in first, second and third line - Results from the prospective German TLK cohort study. Lung Cancer. 2019;130:216–225.
- Tendler S, Grozman V, Lewensohn R, et al. Validation of the 8th TNM classification for small-cell lung cancer in a retrospective material from Sweden. Lung Cancer (Amsterdam, Netherlands). 2018;120:75–81.
- Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer – what limits limited disease? Lung Cancer. 2002;37(3):271–276.
- Bernhardt D, Aufderstrasse S, Konig L, et al. Impact of inflammatory markers on survival in patients with limited disease small-cell lung cancer undergoing chemoradiotherapy. Cancer Manag Res. 2018;10:6563–6569.
- Anami S, Doi H, Nakamatsu K, et al. Serum lactate dehydrogenase predicts survival in small-cell lung cancer patients with brain metastases that were treated with whole-brain radiotherapy. J Radiat Res. 2018;60(2):257–263.
- Jager KJ, van Dijk PC, Zoccali C, et al. The analysis of survival data: the Kaplan–Meier method. Kidney Int. 2008;74(5):560–565.
- Pietanza MC, Byers LA, Minna JD, et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res. 2015;21(10):2244–2255.
- Garassino MC, Torri V, Michetti G, et al. Outcomes of small-cell lung cancer patients treated with second-line chemotherapy: a multi-institutional retrospective analysis. Lung Cancer. 2011;72(3):378–383.
- Jones GS, McKeever TM, Hubbard RB, et al. Factors influencing treatment selection and 30-day mortality after chemotherapy for people with small-cell lung cancer: an analysis of national audit data. Eur J Cancer. 2018;103:176–183.
- Kim YH, Mishima M. Second-line chemotherapy for small-cell lung cancer (SCLC). Cancer Treat Rev. 2011;37(2):143–150.
- Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: a systematic review with meta-analysis. Cancer Biomark. 2016;16(3):415–423.
- Meert AP, Paesmans M, Berghmans T, et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2001;1(1):5.
- Gaspar LE, Gay EG, Crawford J, et al. Limited-stage small-cell lung cancer (stages I–III): observations from the National Cancer Data Base. Clin Lung Cancer. 2005;6(6):355–360.
- Simon GR, Turrisi A. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3):324s–339s.
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116–1125.
- Rossi A, Sacco PC, Sgambato A, et al. Optimal drugs for second-line treatment of patients with small-cell lung cancer. Expert Opin Pharmacother. 2016;17(7):969–976.
- Huang R, Wei Y, Hung RJ, et al. Associated links among smoking, chronic obstructive pulmonary disease, and small cell lung cancer: a pooled analysis in the International Lung Cancer Consortium. EBioMedicine. 2015;2(11):1677–1685.