Abstract
Background: The aim was to describe the incidence and mortality of Hodgkin lymphoma (HL) in Finland in 1996–2015 including classic Hodgkin lymphoma (cHL) subtypes and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL).
Material and Methods: This study included 2851 HL cases registered in the population-based Finnish Cancer Registry between 1996 and 2015. All not otherwise specified (NOS) morphology codes were manually checked and re-coded into cHL subtypes or NLPHL according to the International Classification of Diseases for Oncology 2011, if possible. Thereafter, we analyzed the incidence and mortality of HL by age, gender and time trends and by subtypes.
Results: According to our registry-based study, the incidence of HL was increasing with a 5-year rate of change of 0.3% (95% confidence interval 0.2–0.5), and the mortality was decreasing with –2.8% (95%CI –3.8 to –1.8) correspondingly. The incidence of nodular sclerosis (NS) was 1.57/100 000 person years (n = 1529) and the incidence and mortality remained constant over 1996–2015. The incidence of mixed cellularity (MC) was 0.32/100 000 (n = 453) and it was decreasing with –2.2% (95%CI –3.7 to –0.5), yet the mortality was increasing with 2.7% (95%CI 1.9–3.6). The incidence of NLPHL was 0.29/100 000 accounting for 13% of all HL diagnoses (n = 374), and the incidence and mortality remained constant over the study period. The incidence of lymphocyte-rich (LR) subtype was 0.20/100 000 (n = 252) and remained constant while the mortality decreased. There were only 30 cases of lymphocyte depletion (LD) HL. In this study, 36% of all HL patients were over 50 years old.
Conclusion: The incidence of HL is slightly increasing and the mortality is decreasing in Finland. NLPHL represents 13% of all HL cases in Finland. Over one third of HL patients are over 50-year-old.
Background
Hodgkin lymphoma (HL) is a hematological malignancy originating from the immune system. HL is one of the most common cancers in young adults in the developed Western countries where the highest incidence rates are typically observed at the age of 15–35 and over 55 years. In Finland, HL constitutes 0.6% of all cancers in men and women with approximately 180 new cases per year, but even 8.4% in the age group of 15–35 years [Citation1,Citation2]. The age-standardized (WHO world standard population) incidence of HL is higher in Finland compared to all Nordic countries, 3.0/100 000 person-years versus 2.6/100 000 in men and 2.5/100 000 versus 2.1/100 000 in women. Mortality is at the same level as in all Nordic countries, around 0.3/100 000 in men and 0.2/100 000 in women, respectively [Citation3–5].
HL can be classified into five different subtypes with different morphology. The classic HL (cHL) includes nodular sclerosis (NS), mixed cellularity (MC), lymphocyte-rich (LR) and lymphocyte depletion (LD) subtypes. NLPHL was first recognized as it’s own disease entity in the revised European-American classification of lymphoid neoplasms (REAL) in 1994 and was later on separated from the classic HL subtypes as ‘non-classical’ [Citation6]. It is distinguished from classic HL by its rarity (only 5% of all HL), different clinical and histological characteristics, and by more indolent behavior. According to literature, NLPHL occurs generally at 30–40 years of age and predominantly in men [Citation7–12].
Epidemiological information on different cHL subtypes has not been studied nationally in Finland, since the International Classification of Diseases for Oncology (ICD-O-3) was adopted by the Finnish Cancer Registry (FCR) in 2007. Until that time, all HL cases were coded by the FCR as not otherwise specified (NOS). Thereafter, all HL cases were recoded backwards from years 2000–2006.
Subtype-specific information on the epidemiology of cHL and NLPHL has been reported earlier from Norway (1971–1995) [Citation13], Sweden (2000–2013 [Citation14], 1973–2009 in over 19-year-olds [Citation15], and 1985–2009 in children [Citation16]) and Iceland (1955–1982) [Citation17]. Additionally, in Finland NLPHL cases in 1953–2009 were searched using text mining from pathology notifications in order to study the familial clustering of NLPHL [Citation8].
Information on the incidence and mortality trends of cHL subtypes and NLPHL is important in order to meet the demands of treatment and in search for the etiologic factors contributing to HL development. The aim of this study was to describe the incidence and mortality of Hodgkin lymphoma by age, sex, different cHL subtypes and NLPHL over the past two decades in Finland.
Material and methods
We obtained our data from the Finnish Cancer Registry (FCR), which keeps the national population-based registry on all cancer diagnoses given in Finland since 1953. All hospitals, pathology laboratories and health care professionals are obliged to report new cancer diagnoses to FCR. The reports include information about the patient and the cancer case, such as date of diagnosis, primary site, histology, method of diagnosis, and spreading. The quality of FCR data was recently analyzed by Leinonen et al. and the completeness of HL was estimated at 93.4%, and 99.7% of HL cases were morphologically verified [Citation18].
All HL cases in the FCR from years 1996–2015 with Hodgkin NOS morphology (M9650/3) were re-coded to match the current morphology classification criteria, the International Classification of Diseases for Oncology 2011 (ICD-O-3.1) [Citation19] We re-coded cases into subtypes only if the subtype was sufficiently clearly spelled out in the pathology or clinical report. An oncologist specialized in lymphomas was consulted in unclear cases. In all, 68 full original pathology reports were ordered from district pathology laboratories to obtain more information in unclear cases. Tissue samples were not histologically re-analyzed in this study.
The data were classified into entities based on the morphology code of ICD-O-3.1 [Citation19], which includes the recommendations made in the WHO Classification of Tumors of the Hematopoietic and Lymphoid Tissue in 2008 [Citation20]. Guidelines made for European cancer registries such as HAEMACARE Manual for Coding and Reporting Haematological Malignancies 2008, and European Network of Cancer Registries (ENCR) recommendations were applied in our study [Citation21,Citation22]. The morphology codes M9663/3 Nodular sclerosis, M9652/3 Mixed cellularity, M9651/3 Lymphocyte-rich, M9653/3 Lymphocyte depletion, M9659/3 NLPHL and M9650/3 Hodgkin NOS were used in re-coding and sub-typing cases. The HAEMACARE working group recommends that the nodular sclerosis subtype is coded solely as M9663/3 (Nodular sclerosis NOS), although more specific codes exist in the ICD-O-3.1 (M9664/3 Nodular sclerosis cellular phase, M9665/3 Nodular sclerosis grade 1, M9667/3 Nodular sclerosis grade 2). This resulted in changing 4 cases of M9665/3 and 4 cases of M9667/3 morphology codes in to M9663/3. Also, a total of 5 patients had two different HL diagnoses, and only the first one was taken in our study.
Age and sex-specific incidence and mortality rates were calculated in four five-year calendar periods. Incidence rates were age-standardized using the WHO world standard population. We used 5-year age strata in calculating the age-specific rates, and in addition, to analyze changes in the age-specific incidence over time we used stratification to three age groups (0–14, 15–44 and 45+), respectively.
The average annual percent change (APC) and the corresponding 95% confidence interval (CI) were calculated using log-linear regression analysis as well as joint-point regression, which identifies specific shifts in trends. The Davies test was used for selection of breakpoints. Differences between the groups of median age were tested using a Mann-Whitney U test. Statistical analyses were all done using the statistical software R, version 3.6.0.
Results
Patient characteristics
This study included all HL cases diagnosed in Finland and reported to the FCR between years 1996 and 2015 resulting in a total of 2851 patients (); 1586 males (56%) and 1265 females (44%) with a median age of 37 (range 5–97), 41 years in males and 33 in females, respectively (). Of all HL patients, 36% (n = 1013) were over 50 years of age at diagnosis.
Table 1. Incidence, mortality and the estimate of percent annual change in classic Hodgkin lymphoma subtypes and nodular lymphocyte predominant Hodgkin lymphoma in Finland in 1996–2015.
Table 2. Median age and the proportion of over 50-year old patients in each Hodgkin lymphoma subtype in total and by gender.
Incidence of HL by age, gender and time trends
In 1996–2015, there were from 124 to 182 new HL diagnoses per year (median 139). During the study period, the incidence of HL was higher in males (2.76/100 000 person-years) than in females (2.34/100 000 person-years) (data not shown). There was a small, yet statistically significant, increase in the incidence of HL during the study period (5-year rate of change 0.3%; 95% CI 0.2–0.5) (). When stratified by gender, the increase in incidence was significant only in males (data not shown).
The age-incidence curve has a bimodal appearance with the first peak in the young ages and a second one in the elderly (). The highest age-specific incidence of HL was observed in the age group of 20–24 years, where the incidence in females and males was 6.2 and 5.4 (per 100 000 person-years), respectively. The second peak in the elderly is wider than the first one and differs between males and females. In males, the second peak is divided into two peaks at the age groups of 60–64 and 75–79 years. In females, the second peak is wide and comes in the age group of 70–84 years ().
Figure 1. Age-specific incidence and mortality of Hodgkin lymphoma (all), nodular sclerosis, mixed cellularity and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) in Finland.
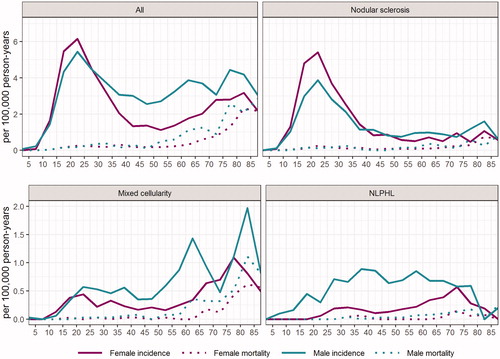
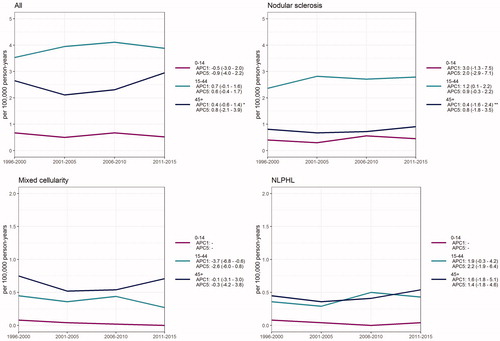
The age-specific incidence of HL did not show any significant change over time in 1996–2015 in Finland when stratified by age into groups of 0–14, 15–44 and 45+ years. At the age group of 45+ years, a breakpoint was found (year 2004) after which the 1-year APC was +4.8% (95%CI 2.7–7.0) ().
Figure 2. Time trends in the incidence of Hodgkin lymphoma (all), nodular sclerosis, mixed cellularity and nodular lymphocyte predominant Hodgkin lymphoma in children (0–14), adults (15–44) and older population (45+) over 1996–2015 in Finland.
APC1: 1-year estimate of percent annual change in incidence with 95% confidence interval in brackets. APC5: 5-year estimate of percent annual change in incidence with 95% confidence interval in brackets. *A breakpoint was found using the Davies test. APC was calculated using joinpoint regression analysis (breakpoint 2004). 2004–2015 APC1 + 4.8% (2.7–7.0). **A breakpoint was found using the Davies test. APC was calculated using joinpoint regression analysis (breakpoint 2004). 2004–2015 APC1 + 5.9% (2.0–10.0). APC values are missing for children in mixed cellularity and NLPHL due to too small number of patients in this age group.
Incidence by cHL subtypes
The most common cHL subtype was NS (54%), with the incidence rate of 1.57/100 000 person-years (1.40 in males and 1.75 in females, respectively) (, ). The incidence of NS remained constant over the study period. NS was observed mostly in young patients with only 19% being over 50 years old. The median age of NS patients was 30 years in males and 27 years in females, respectively, and the difference between median ages was statistically significant (p < .001) (). Interestingly, NS is more common in females at young ages but the proportion of males grows larger at old ages (). The age-specific incidence of NS increased in the age group of 15–44 years (1-year APC +1.2, 95% CI 0.1–2.2) during the study period when stratified by age groups 0–14, 15–44 and 45+ years. At the age group of 45+ a breakpoint was found (year 2004) after which the 1-year APC was +5.9% (95%CI 2.0–10.0) ().
Figure 3. Incidence of classic Hodgkin lymphoma subtypes and nodular lymphocyte predominant Hodgkin lymphoma according to time trends in 1996–2015.
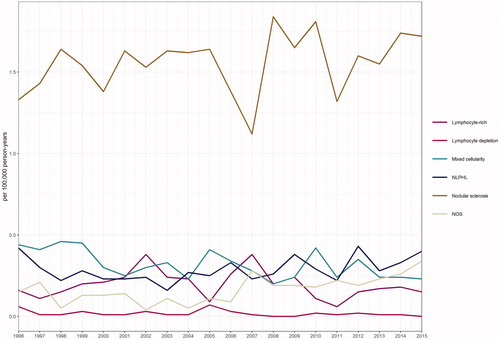
The second most common subtype was MC with the incidence of 0.32/100 000 person-years (0.41 in males and 0.24 in females, respectively) () and it was more common in males almost at all ages (). MC was mainly presented in the elderly patients with 59% of the patients being over 50 years old (). There was a statistically significant decrease in the incidence of MC during the study period (APC –2.1%, 95% CI –3.7 to –0.5) (, ). When stratified by gender, the decreasing trend was statistically significant only in females (data not shown). Age-specific incidence rates showed a decreasing trend at the age group of 15–44 years (1-year APC –3.7%, 95% CI –6.8 to –0.6) while there was no significant change in older population ().
The incidence of LR subtype was 0.20/100 000 person-years (0.28 in males and 0.11 in females, respectively), and it did not change significantly during the study period (, ). The median age in males and females was 45 years and 57 years, respectively (p < .001) ().
The lowest number of new cases was observed in LD subtype throughout the whole study period and the incidence was slowly decreasing (5-year rate of change –6.0%, 95% CI –10.2 to –1.6) (, ). When stratified by gender this decrease was statistically significant only in males (data not shown). 70% of the patients were over 50 years of age with the highest median age both in males (64 years) and in females (66 years) compared to all other subtypes ().
Of all HL diagnoses, 7,5% (n = 213) were classified as Hodgkin NOS during the study period. The incidence of Hodgkin NOS remained constant during the study period ().
Mortality of HL by age, gender and time trends
HL mortality was 0.25/100 000 person-years during the study period in Finland (0.31 in males and 0.2 in females, respectively). There was a statistically significant decrease in mortality both in the 1-year (APC –3.0%, 95% CI –5.0 to –0.9) and 5-year (–2.8%, 95%CI –3.8 to –1.8) rate analysis. () The decrease in mortality was statistically significant both in females and in males. Mortality in men was constantly a bit higher throughout the study period and decreased with a slower rate compared to women (data not shown). Age-specific mortality started to increase after the age of 50 in males and after the age of 60 in females ().
Mortality by cHL subtypes
Mortality from the NS subtype was 0.1/100 000 person-years and there was a decreasing trend over the study period although not statistically significant (). When stratified by gender, mortality from NS decreased more rapidly in males, but the decrease still remained non-significant (data not shown). The age-specific mortality remained substantially low at all ages and both genders ().
Mortality from MC was 0.05/100 000 person-years and it was slowly increasing during 1996–2015 (5-year rate of change 2.7%, 95% CI 1.9–3.6), but the increase was statistically significant only in males (data not shown). Age-specific mortality started to increase after the age of 55–60 in males and after 60–65 in females, was constantly higher in males and reached the highest rates in 80–85-year-old males (1.11/100 000 person-years) ().
In the LR subtype, mortality was 0.03/100 000 and it decreased significantly in both genders over time (5-year rate of change –6.4%, 95% CI –9.8 to –2.9) ().
The mortality to incidence ratio of LD was 0.5 (0.01 versus 0.02 per 100 000 person-years), which indicates that LD is an aggressive cHL subtype. There was no change in the mortality trends of LD subtype during the study period ().
Incidence and mortality of NLPHL
NLPHL accounted for 13% of all HL cases (n = 374) in Finland reaching MC in incidence by 2011–2015 (). There were no statistically significant changes in the net or gender-specific incidence rates (). Age-specific incidence rates showed an increasing trend in the age groups of 15–44 and 45+ years although the APC remained statistically non-significant (). NLPHL was much more common in men than in women (76% males) and men were diagnosed at earlier ages than women with a median age of 45 years in males and 60 years in females (p < .001), respectively (). Mortality from NLPHL remained stable and substantially low in all age groups and in both genders during the study period (, ).
Discussion
The incidence of HL has been slowly increasing over time while HL mortality has been steadily decreasing in Finland during 1996–2015. The proportion of NLPHL was 13% of all HL cases, which is in good coherence with an earlier study made from Finland [Citation8] – yet clearly higher than reported from other Western countries [Citation1,Citation10,Citation23–25].
NS was the most common subtype of cHL as reported in earlier studies [Citation25,Citation26]. The net or gender-specific incidence of NS did not show any significant change during the study period but instead, the age-specific incidences rates increased in the age group of 15–44 years, and after year 2004 also in the age group of 45+ years. Interestingly, the incidence of MC was decreasing but mortality was increasing, which could be explained by the fact that elderly males had the highest age-specific incidence rates and 59% of patients were over 50 years of age (). According to our study and the report made previously by Saarinen et al. [Citation8], the incidence of NLPHL is clearly higher in Finland compared to reports from other Western countries, and remained constant during the study period suggesting that this is a true finding (). Interestingly, a report from France by Laurent et al. found a 10,2% proportion of NLPHL [Citation27] and furthermore Glaser et al. reported that the incidence of NLPHL is increasing in the United States [Citation25]. The reason for the differences between these findings remains yet unknown. There is a germline mutation originating in Finland, which causes a high familial risk of NLPHL, but this is hardly the sole explanation for the high incidence, as found also previously [Citation8]. In our study, 76% of NLPHL patients were males, the incidence was highest at 35–45 years of age, and the mortality rates were constantly very low, all of these being in good coherence with earlier studies [Citation7–12].
The age-incidence curve of HL remained bimodal in shape but the second peak in males was nearly as high as the first peak (incidence 5.4/100 000 at 20–24 years and 4.4/100 000 at 75–79 years) indicating that HL is diagnosed in elderly males also (). This is clearly in contrast to an earlier report made from Nordic countries over 1978–1997 (Hjalgrim et al.) [Citation28] where the first peak in the bimodal curve increased in magnitude and even shifted toward younger ages, whereas the incidence decreased in over 40-year-olds. In our study, the incidence of HL increased by annual rate of change of +4.8% in the age group of 45+ years during the past decade (2004–2015). In other age groups there were no statistically significant changes nor breakpoints found in our analysis ().
In our study, 36% of all the HL patients were over 50 years of age, which is in good coherence with earlier studies [Citation14,Citation29]. This is important since elderly patients are known to have worse prognosis and there are more limitations to treatment options; aggressive treatments are less tolerated because of weaker physical condition of the patient [Citation14,Citation29]. Even though the proportion of elderly patients is increasing the mortality of HL is decreasing steadily, which could indicate improved diagnostics in Finland enabling identification of accurate subtypes, risk stratification and stage, and based on this information also improved treatment options. However, mortality of MC subtype is increasing reflecting challenges in the treatment of elderly patients with HL.
The strengths of our study include the use of nationwide population-based cancer registry data, where pathology reports are received from all hospital districts, private practices and pathology laboratories all over Finland. All patients diagnosed with cancer are reported to the FCR by law, as is also the information from death certificates. An individual level linkage system enables national cause of death surveillance. Hence the mortality rates calculated from the FCR data are highly reliable and there are no losses to follow up.
Registry data is known to have its limitations, which should be noticed when analyzing the results of our study [Citation30]. First, we did not perform any histological re-analysis of tissue samples but relied on the information sent to the FCR (pathology and clinical reports) when re-coding HL NOS morphology codes into HL subtypes. In unclear cases the full original pathology reports were ordered. Second, the pathologists who had analyzed tissue samples over the years were not all specialists of hematopathology, which is known to affect the accuracy of the correct diagnosis [Citation7,Citation11,Citation31,Citation32]. Third, a clear limitation in analyzing HL subtypes (especially when stratified further by age, gender and time) is the small number of patients due to HL being a rare disease in Finland with good survival (83% and 84% in women and men, respectively). Statistical errors are prone to occur when small data is stratified too far, and caution should be used when interpreting such results.
The role of Epstein-Barr virus (EBV) in the pathogenesis of HL is being actively investigated worldwide and it has been even suggested that EBV status should be included in the classification of cHL [Citation33–35]. In this registry-based study we did not have the EBV status, as it was not routinely included in the pathology reports during the study period. Neither was long-term treatment toxicity analyzed in our study. Further studies on pan Nordic data are warranted to study subtype specific data, such as EBV status or survival.
Surprisingly many HL NOS codes were present also in the most recent years. We were able to recode only 35 Hodgkin NOS morphology codes out of 151 from years 2007 to 2015 (when the ICD-O-3 was used at the FCR). The reason stated for this in the original pathology report was that the biopsy material was insufficient or it included two classification choices. It could be possible that it is difficult to interpret the specific subtypes of the elderly patients’ HL representing an increasing proportion of the specimen (65% of HL NOS patients were over 50 years of age). Single coding errors were also noticed. We did not notice any unexpected or deviated changes in the incidence and mortality rates around year 2007, when the classification criteria changed in the FCR.
As the immunohistochemical approaches improve, the diagnostics and categorizing HL into different subtypes become more and more specific. Hence it is important for clinicians and pathologists to know the current trends in the epidemiology of different cHL subtypes and NLPHL. The classification criteria for HL did not change in the 2016 revision of the World Health Organization classification of lymphoid neoplasms [Citation36], but more specific information on the possible features of NLPHL and lymphocyte-rich HL was added. It is emphasized that classification to different cHL subtypes and NLPHL is important because they differ from each other by clinical and prognostic features and can affect treatment choices. Therefore we suggest co-work in the future between Nordic countries to add data for more complex analysis on cHL subtypes and NLPHL.
Acknowledgements
We thank the staff of Finnish Cancer Registry for practical assistance in conducting this study and pathologists around Finland who were consulted in unclear cases.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bazzeh F, Rihani R, Howard S, et al. Comparing adult and pediatric Hodgkin lymphoma in the Surveillance, Epidemiology and End Results Program, 1988–2005: an analysis of 21 734 cases. Leuk Lymphoma. 2010;51(12):2198–2207.
- Kharazmi E, Fallah M, Pukkala E, et al. Risk of familial classical Hodgkin lymphoma by relationship, histology, age, and sex: a joint study from five Nordic countries. Blood. 2015;126(17):1990–1995.
- Finnish Cancer Registry [Internet]. Finland: Finnish cancer registry; 2016. [cited 2019 May 14]. Available from: https://cancerregistry.fi/statistics/cancer-statistics/.
- Danckert B, Ferlay J, Engholm G, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Counties, Version 8.2 [updated 2019 March 26; cited 2019 May 14]. Association of the Nordic Cancer Registries. Denmark: Danish Cancer Society. Available from: http://ancr.nu.
- Storm HH, Klint A, Tryggvadottir L, et al. Trends in the survival of patients diagnosed with malignant neoplasms of lymphoid, haematopoietic, and related tissue in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49(5):694–712.
- Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361–1392.
- Strobbe L, Valke LL, Diets IJ, et al. A 20-year population-based study on the epidemiology, clinical features, treatment, and outcome of nodular lymphocyte predominant Hodgkin lymphoma. Ann Hematol. 2016;95(3):417–423.
- Saarinen S, Pukkala E, Vahteristo P, et al. High familial risk in nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol. 2013;31(7):938–943.
- Spinner MA, Varma G, Advani RH. Modern principles in the management of nodular lymphocyte-predominant Hodgkin lymphoma. Br J Haematol. 2019;184(1):17–29.
- Gerber NK, Atoria CL, Elkin EB, et al. Characteristics and outcomes of patients with nodular lymphocyte-predominant Hodgkin lymphoma versus those with classical Hodgkin lymphoma: a population-based analysis. Int J Radiat Oncol Biol Phys. 2015;92(1):76–83.
- Hawkes EA, Wotherspoon A, Cunningham D. The unique entity of nodular lymphocyte-predominant Hodgkin lymphoma: current approaches to diagnosis and management. Leuk Lymphoma. 2012;53(3):354–361.
- Lee AI, LaCasce AS. Nodular lymphocyte predominant Hodgkin lymphoma. Oncologist. 2009;14(7):739–751.
- Foss Abrahamsen A, Egeland T, Hansen S, et al. Hodgkin’s disease in a national and hospital population: trends over 20 years. Eur J Cancer. 1997;33(14):2380–2383.
- Bjorkholm M, Weibull CE, Eloranta S, et al. Greater attention should be paid to developing therapies for elderly patients with Hodgkin lymphoma-A population-based study from Sweden. Eur J Haematol. 2018;101:106–114.
- Sjoberg J, Halthur C, Kristinsson SY, et al. Progress in Hodgkin lymphoma: a population-based study on patients diagnosed in Sweden from 1973–2009. Blood. 2012;119:990–996.
- Englund A, Hopstadius C, Enblad G, et al. Hodgkin lymphoma–a survey of children and adolescents treated in Sweden 1985–2009. Acta Oncol. 2015;54(1):41–48.
- Agnarsson BA, Olafsdottir K, Benediktsson H. Tumours in Iceland. Hodgkin’s disease and non-Hodgkin’s malignant lymphomas. A histological classification and epidemiological considerations. Acta Pathol Microbiol Immunol Scand A. 1987;95:23–28.
- Leinonen MK, Miettinen J, Heikkinen S, et al. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31–39.
- International Agency for for Research on Cancer and World Health Organization. International Classification of Diseases for Oncology ICD-O-3 online. 2011. Accessed 2019 June 16.
- Swerdlow SH, Campo E, Harris N, et al. WHO classification of tumours of haematopoietic and lymphoid tissue. Geneva: World Health Organisation; 2008.
- Gavin A, Rous B, Marcos-Gragera R, et al. European Network of Cancer Registries. Towards optimal clinical and epidemiological registration of haematological malignancies: guidelines for recording progressions, transformations and multiple diagnoses. Eur J Cancer. 2015;51(9):1109–1122.
- Sant M, Karjalainen-Lindsberg M, Maynadie M. HAEMACARE Manual for Coding and Reporting Haematological Malignancies 2010.
- Nogova L, Reineke T, Brillant C, et al. German Hodgkin Study Group. Lymphocyte-predominant and classical Hodgkin’s lymphoma: a comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol. 2008;26:434–439.
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276.
- Glaser SL, Clarke CA, Keegan TH, et al. Time trends in rates of Hodgkin lymphoma histologic subtypes: true incidence changes or evolving diagnostic practice? Cancer Epidemiol Biomarkers Prev. 2015;24(10):1474–1488.
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132.
- Laurent C, Baron M, Amara N, et al. Impact of expert pathologic review of lymphoma diagnosis: study of patients from the French Lymphopath Network. J Clin Oncol. 2017;35(18):2008–2017.
- Hjalgrim H, Askling J, Pukkala E, et al. Incidence of Hodgkin’s disease in Nordic countries. Lancet. 2001;358(9278):297–298.
- Evens AM, Sweetenham JW, Horning SJ. Hodgkin lymphoma in older patients: an uncommon disease in need of study. Oncology. 2008;22(12):1369–1379.
- Pukkala E, Engholm G, Hojsgaard Schmidt LK, et al. Nordic cancer registries – an overview of their procedures and data comparability. Acta Oncol. 2018;57(4):440–455.
- Proctor IE, McNamara C, Rodriguez-Justo M, et al. Importance of expert central review in the diagnosis of lymphoid malignancies in a regional cancer network. J Clin Oncol. 2011;29(11):1431–1435.
- Stevens WB, van Krieken JH, Mus RD, et al. Centralised multidisciplinary re-evaluation of diagnostic procedures in patients with newly diagnosed Hodgkin lymphoma. Ann Oncol. 2012;23(10):2676–2681.
- Zhang T, Fu Q, Gao D, et al. EBV associated lymphomas in 2008 WHO classification. Pathol Res Pract. 2014;210(2):69–73.
- Urayama KY, Jarrett RF, Hjalgrim H, et al. Genome-wide association study of classical Hodgkin lymphoma and Epstein-Barr virus status-defined subgroups. J Natl Cancer Inst. 2012;104(3):240–253.
- Vockerodt M, Yap LF, Shannon-Lowe C, et al. The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol. 2015;235(2):312–322.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390.
