Abstract
Background: There are concerns about timely access to appropriate cancer treatment for the growing immigrant population in Norway. This study aims to compare waiting times between cancer diagnosis and start of cancer treatment, as well as treatment patterns between immigrants in Norway and the host population.
Material and methods: We performed a nationwide, registry-based study with individual-level data, including 213,320 Norwegians and 8324 immigrants diagnosed with breast, colorectal, lung or prostate cancer in 1990–2014. Differences in time from diagnosis to treatment and in treatment patterns were described for the selected cancer sites. The Cox and logistic regressions were used to adjust for patient and tumour characteristics.
Results: After adjustment for covariates, hazard ratios for time from diagnosis to treatment for non-Western immigrants compared to Norwegians were 0.88 (95% confidence interval (CI): 0.82–0.95) for breast cancer and 0.84 (95% CI: 0.75–0.95) for lung cancer, indicating longer waiting times. Treatment patterns in the four major cancer sites were similar among immigrants and the Norwegian host population, except for breast cancer, where women from East and South Asia received less breast-conserving surgery than the Norwegian host population (adjusted odds ratios 0.65 (95% CI: 0.46–0.93) for East Asians and 0.75 (95% CI: 0.50–1.13) for South Asians).
Conclusions: The present study reports delayed treatment for lung and breast cancer among immigrants from non-Western countries in Norway. Systematic differences in cancer treatment were not detected. However, less breast-conserving surgery among breast cancer patients from Asia compared to Norwegians was observed.
Introduction
In 2017, first-generation immigrants comprised 13.8% of the Norwegian population [Citation1]. Studies have shown that immigrants interact differently with health care systems, raising concerns about timely access to appropriate treatment in this population [Citation2–5]. To reduce waiting times, policy makers have implemented several measures, including standardised patient pathways, waiting time guarantees and provider competition [Citation6]. The Norwegian health care system aims to provide equity in health, thus one might expect waiting times for severe diseases like cancer to be equal across population groups [Citation7]. However, waiting time guarantees, provider competition and other measures could very well generate disparities if there are structural, political or societal factors that exclude immigrants from these benefits. In a European context, very little is known about waiting times after cancer diagnosis in immigrant populations. A review from 2013, which focussed on ethnic disparities in primary care and diagnostic waiting time intervals, was inconclusive [Citation8]. The present study aims to compare waiting times between cancer diagnosis and start of cancer treatment between immigrants in Norway and the host population.
Choosing a cancer treatment regimen is a multifactorial and complex process; the choice is made based on patient and disease characteristics, availability and cost of treatment options, as well as patient preferences. Physicians follow clinical guidelines for all major cancer sites. In some cases, this implies strict decision algorithms, with few or no alternatives. In other cases, faced with vague guidelines or multiple options, the physician's subjective assessment and the patient’s influence become increasingly important. As described by Luo et al. [Citation9], quality of patient–physician communication, preferences regarding who controls decision-making, and cultural attitudes are all factors that might cause differences in cancer treatment among minority groups. Treatment differences based on ethnicity or birthplace in the United States have been well documented, e.g., for surgical treatment of breast cancer, for treatment of localised prostate cancer, and for use of robot-assisted prostatectomy [Citation10–12]. There is a need for similar analyses among immigrants with cancer in Norway in order to understand differences in treatment preferences, access to new treatments and adherence to clinical guidelines. This study covers multiple cancer sites (breast, colorectal, lung and prostate) and aims to compare waiting times between cancer diagnosis and start of cancer treatment between immigrants in Norway and the host population, and to describe treatment patterns across sites and stage at diagnosis.
Material and methods
Study population
All subjects with breast (C50), colorectal (C18–20), lung and trachea (C33–34) or prostate cancer (C61) diagnosed in the period 1990–2014 were identified from the population-based Cancer Registry of Norway. Subjects with a cancer diagnosis prior to the study period (n = 11,343), and cases registered based on death certificate only or detected through autopsy were excluded (n = 2729). For individuals with multiple cancer diagnoses (n = 31,432), only the first cancer diagnosis was included. An immigrant was defined as someone born abroad whose parents were also born abroad. With exception of second-generation immigrants, the remaining population was categorised as Norwegian, i.e., as part of the host population.
Data sources and study variables
The following patient and tumour characteristics were obtained from the Cancer Registry of Norway: cancer site, age at diagnosis, sex, place of residence at diagnosis (county), status (alive, deceased, emigrated), status date, stage at diagnosis, date of diagnosis, information about surgery, and days from date of diagnosis to surgery and start of radiotherapy (radiotherapy information was available from 1997). As defined by Aas et al., non-metastatic prostate cancer patients who had not received any local treatment were considered to be on active surveillance or watchful waiting [Citation13].
The following information was obtained from Statistics Norway: country of origin, immigration status, marital status, personal income (low: <20th percentile, middle: 20–80th percentile and high: >80th percentile, adjusted for year and sex) and education level (low: <10 years, middle: 10–12 years and high: >12 years of education). Countries of origin were categorised into three Western and nine non-Western geographic regions (see online supplement 1). The level of categorisation used in each analysis was based on the number of available immigrant cases. Data on marital status, personal income and education level were obtained for the year prior to diagnosis.
From the National Patient Registry (data available from 2008), we obtained an adjusted Charlson comorbidity index (CCI) constructed with one year of historic data [Citation14,Citation15]; national medical, surgical and procedural treatment codes linked to a cancer diagnosis; and associated admission dates. The treatment codes were used to construct the following variables: chemotherapy treatment, time from diagnosis to admission for chemotherapy treatment, neoadjuvant and adjuvant chemotherapy for breast cancer, laparoscopic surgery for colorectal cancer, and robot-assisted prostatectomy. Detailed definitions and availability of each outcome variable are described in online supplement 2.
Data sources were linked using the unique personal identification number assigned to all Norwegian citizens and immigrants with legal residence status. The identification number was replaced with a project-specific identification number before distribution of the data to the researchers.
Statistical analysis
Time-to-event methodology was used to analyse waiting times. The Cox regression was applied to estimate the hazard ratio of receiving treatment between immigrants and the host population. A hazard ratio below one indicated an increased risk of receiving treatment later than the reference group (host population). Subjects were censored upon death or at the end of the study period. Logistic regression was used to estimate the odds ratios for receiving a specific treatment. Three models were applied in the Cox and logistic regressions: an unadjusted univariate model; a core model adjusted for age (categorical variable with 10-year intervals), sex, year of diagnosis (categorical variable), stage at diagnosis, place of residence, and CCI; and a fully adjusted model that also included socioeconomic variables (marital status, personal income and education level). The core model for colorectal cancer included information about cancer subsite (colon or rectum cancer), and for breast cancer it included type of surgery (breast-conserving or mastectomy) when analysing adjuvant treatments. Patients who received treatment before, or at the same time as their cancer diagnosis (acute cases), were excluded from the waiting time analyses (3.4% in breast, 17.2% in colorectal and 7.5% in lung cancer). Waiting times for prostate cancer were not reported, as it is difficult to record start of treatment when the treatment decision can be to simply implement active surveillance. We conducted a sensitivity analysis of waiting times, in which patients not treated within 1 year of diagnosis were excluded. Multiple imputation was used to account for unknown stage at diagnosis, education level, as well as CCI before 2009. Multiple imputation was performed by cancer site using the mice package in R (version 2.25), with 20 imputations and default settings. Variables used in the imputation model included follow-up time, status, basis of the diagnosis, as well as all variables used in the fully adjusted regression models. Stratifications and regression analyses were performed for each of the imputed datasets and combined using Rubin’s Rule [Citation16]. The reported ratios with 95% confidence intervals (CIs) in the text refer to the fully adjusted models with imputed data. Data management and statistical analysis were performed with R (version 3.4.1) [Citation17].
Results
After exclusions, a total of 251,343 subjects with breast, colorectal, lung or prostate cancer were available for analysis (). The complete dataset and the subset with additional National Patient Registry data were similar in terms of patient characteristics (only descriptive statistics for the full dataset shown). A younger mean age at diagnosis was observed among immigrants, particularly non-Western immigrants, than among the host population. This was reflected in the prevalence of the specific cancer sites. Breast cancer was the most frequent cancer site among immigrants, while prostate cancer was most common in the host population. However, the difference in age makes direct comparisons of unadjusted estimates between the population groups misleading. Attention should therefore be directed to adjusted estimates. Both Western and Non-Western immigrants had a higher proportion of persons with a high education level compared to the Norwegian host population. Non-Western immigrants had a higher proportion with low income than the other groups. The immigrant population overall had fewer comorbidities, although after stratification by age, CCIs were similar between the groups.
Table 1. Descriptive statistics.
Waiting times
Overall, median waiting times from diagnosis to start of treatment were 23 days for breast cancer, 27 days for colorectal cancer and 43 days for lung cancer. For lung cancer, there was a tendency towards faster treatment, and a larger proportion of patients were treated in 2011–2014 (). After stratification by age and time period (2008–2010 and 2011–2014), non-Western immigrants with breast and lung cancer appeared to have slightly longer waiting times for treatment following diagnosis than the Norwegian host population. The number of subjects in each strata in are reported in online supplement 3. Further descriptive analysis stratified by other variables (stage, sex and income level) suggest that the delays in lung cancer treatment might be larger in female immigrants above 59 years (available in online supplement 4, 5 and 6).
Figure 1. The Kaplan–Meier curves describing waiting times stratified by age (≥60 and <60) and time period (2008–2010 and 2011–2014). Number of subjects at risk in each strata are available online in supplement 3. Patients are censored upon death and end of the study period.
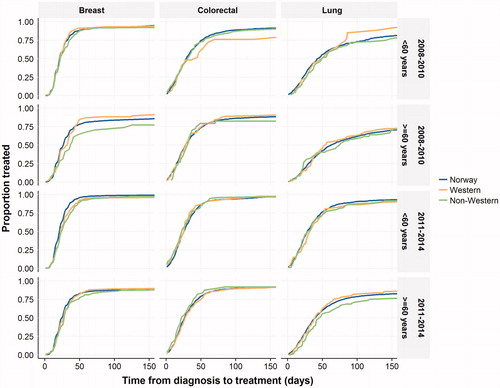
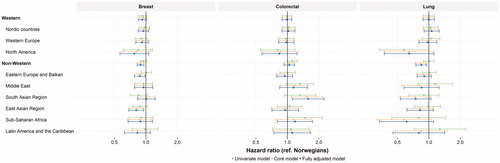
These findings were confirmed in the regression analysis adjusting for available confounders. Non-Western immigrants had hazard ratios for receiving breast cancer treatment of 0.88 (95% CI: 0.82–0.95) and lung cancer treatment of 0.84 (95% CI: 0.75–0.95) ( and online supplement 7). For lung cancer, the subpopulations from sub-Saharan Africa and North America were notable, with hazard ratios of 0.61 (95% CI: 0.33–1.11) and 0.64 (95% CI: 0.37–1.11), respectively. The analysis of waiting times for colorectal cancer showed no clear trend across immigrant groups. Some point estimates were above one (e.g., South Asian Region hazard ratio: 1.58 (95% CI: 1.11–2.24)), while others were below (e.g., Eastern Europe and the Balkans hazard ratio: 0.94 (95% CI: 0.79–1.12)). After excluding patients who did not receive treatment within 1 year of diagnosis, certain point estimates changed somewhat, but this did not alter the interpretation of the data (data not shown).
Figure 2. Hazard ratios estimated by the Cox regression for times from diagnosis to start of treatment for breast, colorectal and lung cancer are defined as the event and a hazard ratio below one indicates longer waiting times for treatment. The core model was adjusted for age, sex (not for breast cancer), year of diagnosis, place of residence, stage at diagnosis, CCI, type of surgery (only for breast cancer) and cancer site (only for colorectal cancer). The fully adjusted model was further adjusted for socioeconomic factors (income, education and marital status). Exact estimates and confidence intervals presented in the figure are available online in supplement 6.
Treatment
Breast cancer treatment
Nearly, all patients with stage I/II/III breast cancer received surgery. In total, 54% of patients with stage I or II breast cancer received breast-conserving surgery (). Stratified analyses of patients receiving breast-conserving surgery by income level and stage are available in online supplement 8. The logistic regression showed that women from the East Asian region, and potentially the South Asian region, received less breast-conserving surgery ( and online supplement 9). The odds ratios were 0.65 (95% CI: 0.46–0.93) for immigrants from the East Asian and 0.75 (95% CI: 0.50–1.13) for the South Asian region. Further, there was a tendency of less use of radiotherapy in some of the non-Western immigrant groups, particularly in immigrants from the Middle East, the South Asian region and Eastern Europe and the Balkans. The point estimates for treatment with chemotherapy had substantial uncertainty, and for non-Western immigrants they were sensitive to adjustment for year of diagnosis. It can be noted that, while immigrants from the Middle East and the South Asian region received less adjuvant radiotherapy, they received more adjuvant chemotherapy compared to the Norwegian host population.
Figure 3. Odds ratios estimated by logistic regression for choice of treatment for (a) breast cancer, (b) colorectal cancer, (c) lung cancer and (d) prostate cancer. An estimate above 1 indicates higher chance of receiving the specific treatments. Estimates and confidence intervals from the fully adjusted models presented in the figure are available online in supplement 9. The core models were adjusted for age, sex, year of diagnosis, place of residence, stage at diagnosis (when relevant), CCI, cancer site (only applicable for colorectal cancer) and type of surgery (only applicable for adjuvant breast cancer treatment). In all analyses, the fully adjusted model represents the core model further adjusted for income, education and marital status.
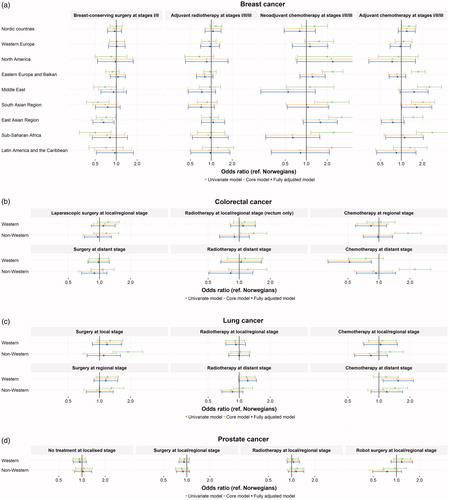
Table 2. Subjects available for each analysis and percentage of all available subjects receiving the respective treatments.
Colorectal cancer treatment
Independent of immigrant status, nearly all patients diagnosed with local or regional stage colorectal cancer were treated with surgery. In total, 22% of surgeries performed on local and regional stage cases were done with laparoscopy (). There were no differences in the use of radiotherapy for local or regional stage rectal cancer ( and online supplement 9). Western immigrants were less likely to receive chemotherapy when diagnosed with distant colorectal cancer (odds ratio: 0.52 (95% CI: 0.32–0.84)).
Lung cancer treatment
The resection rates were 50% for patients diagnosed with local lung cancer, and 27% for those diagnosed with regional lung cancer (). Western immigrants with local and regional lung cancer potentially had a higher resection rate than the host population ( and online supplement 9). For patients with distant metastasis at diagnosis, 39% were treated with radiotherapy and 37% with chemotherapy. Our results also showed higher use of chemotherapy and radiotherapy among Western immigrants than the host population.
Prostate cancer treatment
No differences were observed in treatment patterns for prostate cancer ( and online supplement 9). Point estimates for no local treatment (active surveillance or watchful waiting) and for surgery and radiotherapy among local/regional stage cases were close to unity. The odds ratio for receiving robot-assisted prostatectomy in the non-Western immigrant group was 0.75 (95% CI: 0.49–1.17) compared to the Norwegian host population.
Discussion
Waiting times
The present study reports longer waiting times from diagnosis to start of treatment for non-Western immigrants with breast and lung cancer. As breast cancer patients overall have short waiting times, it is not likely that the observed difference has implications for survival [Citation18,Citation19]. For lung cancer, time to treatment is in general longer, and a hazard ratio of 0.84 translates to a noticeable delay for non-Western immigrants. The clinical relevance of this delay is unknown. However, immigrants in Norway have recently been shown to have good lung cancer survival relative to the host population, making us primarily concerned with the psychosocial implications and the lack of equity in the treatment of this patient group [Citation20].
Unadjusted local variation in waiting times within regions, differences in medical complexity, or a mix of cancer subtypes are all possible explanations for the observed findings [Citation21–23]. If immigrants have more complex diagnoses that are not captured by adjustment for stage at diagnosis, this might make the longer waiting times reasonable. For breast cancer, lower attendance to mammography screening among immigrants might be an additional explanation [Citation24]. Patients diagnosed outside the mammography programme could have a slight delay in time to treatment compared to programme participants. For lung cancer, the findings might reflect the extensive diagnostic evaluation that is often required before treatment can be initiated [Citation23]. The speed with which necessary diagnostic tests are performed could be susceptible to both the patient’s and health care provider’s behaviour, as well as access to professional translators. One issue of concern is that immigrants may seek full or partial treatment in their home countries, and thus can be lost to follow-up. Further, we did not have access to start dates for certain treatments initiated outside the specialised health care system, e.g., oral chemotherapy.
Treatment patterns
The present study reports differences in breast-conserving surgery for Asian immigrants compared to the Norwegian host population, which aligns with findings from the United States [Citation11]. Previously explored reasons include patient attitudes towards not needing to preserve the breast, smaller breast sizes, and fear and cultural beliefs [Citation25]. Further, as a consequence of different screening attendance [Citation24], Norwegians might present with smaller tumour sizes, and therefore may more often be treated with breast-conserving surgery [Citation26]. Although it was associated with uncertainty, we observed less radiotherapy in some of the non-Western immigrant groups with breast cancer. In addition to accompanying breast-conserving and other non-radical surgery, radiotherapy is recommended if the tumour is large or has spread to the lymph nodes (N + disease) [Citation27]. Less use of radiotherapy is not consistent with previous findings that non-Western immigrants in Norway are diagnosed with more advanced breast cancer [Citation5]. Based on this, we would rather expect larger tumour sizes and more N + disease, followed by more radiotherapy, even after adjustment for stage at diagnosis. Successful radiotherapy treatment depends on good communication between physicians and patients, as well as the patient’s motivation or ability to follow the strict preparation and treatment regime. Therefore, less radiotherapy among certain immigrants could reflect communication difficulties between physicians and patients. Interestingly, some of the groups with the least use of adjuvant radiotherapy were shown to be treated more often with adjuvant chemotherapy. A detailed analysis of tumour characteristics and adherence to breast cancer treatment guidelines in immigrants might provide valuable information.
There were some indications that Western immigrants received more resection of primary tumours and palliative treatment for lung cancer. Western immigrants with lung cancer might be a particularly resourceful group, not well described by typical socioeconomic factors. We already know that lung cancer patients are particularly prone to socioeconomic differences [Citation28]. We observed that Western immigrants diagnosed with distant stage colorectal cancer received less chemotherapy. One explanation may be that Western immigrants with colorectal cancer travel to their home country for treatment to a larger extent than other cancer patients.
We analysed use of laparoscopic surgery in colorectal cancer and robot-assisted prostatectomy to explore the use of new treatment techniques in immigrants. Although it was associated with substantial uncertainty, we would like to highlight the point estimate for use of robot-assisted prostatectomy, which indicated that this surgery was used less frequently in non-Western immigrants. We consider this point estimate less uncertain than what is reflected with the wide CI. The finding is likely due to the fact that one of the major hospitals in Norway, with a large proportion of non-Western immigrants in its catchment area, did not offer robot-assisted prostatectomy during the study period.
The purpose of our analyses was not to determine if immigrants were treated optimally, but to understand if treatment patterns differed. A more detailed classification of the diseases could have improved the analyses. It could be argued that the study did not have sufficient power to detect relevant differences in treatment. We aimed to do meaningful analyses at a reasonable level of granularity, in terms of both immigrant groups and stratification of the analyses. With the number of comparisons made, the issue of multiple comparisons is relevant. As a consequence, we have highlighted differences that are consistent across immigrant groups and cancer sites, where we have supporting evidence from the literature or where other plausible explanations for the findings exist. The study is subject to the potential biases of observational, registry-based studies, which have been described elsewhere [Citation29]. Although registry data on immigrants in Norway are considered to be of high quality, certain information bias might be relevant, e.g., for education level [Citation30].
Conclusions
The present study is unique, being the first to describe the events following cancer diagnosis in immigrants, namely waiting times for treatment and treatment patterns. To our knowledge, no comprehensive analysis with high-quality population data has been presented previously.
Our findings indicate that non-Western immigrants wait longer than the Norwegian host population for treatment of breast and lung cancer. For breast cancer, the differences are small, while for lung cancer the delays are longer. The implications of the delay in lung cancer in terms of prognosis and patient satisfaction is unknown. Further, we report differences in breast-conserving surgery for Asian immigrants compared to the Norwegian host population. With the exception of observed differences in the treatment of breast cancer, we did not detect any evidence of systematic differences in treatment between immigrants and the Norwegian host population across the examined cancer sites.
Ethics approval
This study has been approved by the Regional Committee for Medical and Health Research Ethics of the South-East Region in Norway.
Supplemental Material
Download Zip (1.2 MB)Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Sandnes T. Befolkningsgruppe i stadig endring. Statistics Norway Discussion Papers. Statitisk lSentralbyra; 2017; [cited 2017 Aug 24]. Available from: http://ssb.no/befolkning/artikler-og-publikasjoner/befolkningsgruppe-i-stadig-endring
- Schouten BC, Meeuwesen L. Cultural differences in medical communication: a review of the literature. Patient Educ Couns. 2006;64(1–3):21–34.
- Scheppers E, van Dongen E, Dekker J, et al. Potential barriers to the use of health services among ethnic minorities: a review. Fam Pract. 2006;23(3):325–348.
- Diaz E, Calderón-Larrañaga A, Prado-Torres A, et al. How do immigrants use primary health care services? A register-based study in Norway. Eur J Public Health. 2015;25(1):72–78.
- Thøgersen H, Møller B, Robsahm TE, et al. Comparison of cancer stage distribution in the immigrant and host populations of Norway, 1990–2014. Int J Cancer. 2017;141(1):52–61.
- OECD. OECD health policy studies value for money in health spending. Paris: OECD Publishing; 2010.
- Magnussen J, Vrangbaek K, Saltman RB, editors. Nordic health care systems. Recent reforms and current policy challenges. Open University Press McGraw Hill; 2009. p. 3–19.
- Martins T, Hamilton W, Ukoumunne OC. Ethnic inequalities in time to diagnosis of cancer: a systematic review. BMC Fam Pract. 2013;14:197.
- Luo T, Spolverato G, Johnston F, et al. Factors that determine cancer treatment choice among minority groups. J Oncol Pract. 2015;11(3):259–261.
- Kim J, ElRayes W, Wilson F, et al. Disparities in the receipt of robot-assisted radical prostatectomy: between-hospital and within-hospital analysis using 2009–2011 California inpatient data. BMJ Open. 2015;5(4):e007409.
- Chavan S, Goodman M, Jemal A, et al. Receipt of surgical treatment in US women with early stage breast cancer: does place of birth matter? Ethn Dis. 2014;24(1):110–115.
- Shavers VL, Brown ML, Potosky AL, et al. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. J Gen Intern Med. 2004;19(2):146–155.
- Aas K, Axcrona K, Kvåle R, et al. Ten-year mortality in men with nonmetastatic prostate cancer in Norway. Urology. 2017;110:140–147.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Nilssen Y, Strand T-E, Wiik R, et al. Utilizing national patient-register data to control for comorbidity in prognostic studies. Clin Epidemiol. 2014;6:395–404.
- Rubin DB. Multiple imputation for nonresponse in survey. New York: Wiley; 1987.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [cited 2020 Jan 2]. Available from: https://www.R-project.org/
- Caplan L. Delay in breast cancer: implications for stage at diagnosis and survival. Front Public Health. 2014;2:87.
- Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148(6):516–523.
- Thøgersen H, Møller B, Robsahm TE, et al. Differences in cancer survival between immigrants in Norway and the host population. Int J Cancer. 2018;143(12):3097–3105.
- Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–2911.
- Bouche G, Ingrand I, Mathoulin-Pelissier S, et al. Determinants of variability in waiting times for radiotherapy in the treatment of breast cancer. Radiother Oncol. 2010;97(3):541–547.
- Stokstad T, Sørhaug S, Amundsen T, et al. Medical complexity and time to lung cancer treatment – a three-year retrospective chart review. BMC Health Serv Res. 2017;17(1):45.
- Bhargava S, Tsuruda K, Moen K, et al. Lower attendance rates in immigrant versus non-immigrant women in the Norwegian Breast Cancer Screening Programme. J Med Screen. 2018;25(3):155–161.
- Pham JT, Allen LJ, Gomez SL. Why do Asian-American women have lower rates of breast conserving surgery: results of a survey regarding physician perceptions. BMC Public Health. 2009;9(1):246.
- Chagpar AB, Studts JL, Scoggins CR, et al. Factors associated with surgical options for breast carcinoma. Cancer. 2006;106(7):1462–1466.
- Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av pasienter med brystkreft [National guidelines for diagnosis and treatment of patients with breast cancer]. Oslo, Norway: The Norwegian Directorate of Health; 2017.
- Nilssen Y, Strand T-E, Fjellbirkeland L, et al. Lung cancer treatment is influenced by income, education, age and place of residence in a country with universal health coverage. Int J Cancer. 2016;138(6):1350–1360.
- Thygesen LC, Ersbøll AK. When the entire population is the sample: strengths and limitations in register-based epidemiology. Eur J Epidemiol. 2014;29(8):551–558.
- Vassenden K. The data basis for Statistics Norway’s migration-related statistics. Statistics Norway; 2010. [cited 2020 Jan 2]. Available from: http://www.ssb.no/a/english/publikasjoner/pdf/sa122/data_basis.pdf
