Introduction
Anatomical surgical resection is recommended for early-stage non-small cell lung cancer (NSCLC) in medically operable patients [Citation1,Citation2]. Stereotactic ablative radiotherapy (SABR) or hypo-fractionated high-dose radiotherapy are recommended for patients who are unfit for surgery or at high-risk of complications, or for medically operable patients unwilling to accept the operative risks [Citation1–7]. Multiple retrospective matched comparisons of surgery and SABR in operable patients have been published, but so far, attempts at a prospective randomized study have been unsuccessful [Citation8,Citation9]. With these options, it is conceivable that treatment strategies might differ between hospitals, depending, for example, on the composition of their multidisciplinary tumor board and the access to on-site lung cancer surgery and radiotherapy. In 2007, the Quality of Cancer Care taskforce of the Dutch Cancer Society concluded that the quality of care could be improved by concentrating complex services in specialized settings with adequate resources, expertise, and volume. This recommendation contributed to a reduction in hospitals with in-house lung cancer surgery from 79 in 2005 to 43 in 2015 [Citation10–13]. We tested the hypothesis that patterns of care (i.e. preference for surgery) and survival would be different in hospitals with and without in-house lung cancer surgery.
Material and methods
The Netherlands cancer registry
The Netherlands Cancer Registry (NCR) collects data on all cancer patients diagnosed in the Netherlands. It is notified of newly diagnosed malignancies by the national automated pathological archive and of hospital discharge diagnoses. Information on demographics, diagnosis, staging, and treatment is extracted from medical records in all 77 Dutch hospitals by NCR personnel. Survival status is updated annually via a computerized link with the national civil registry.
Study population
Information on 11,847 patients diagnosed with stage I NSCLC from 1 January 2012 to 31 December 2016 was retrieved from the NCR, after approval by the Privacy Review Board. The study population consisted of patients who had either radiotherapy or surgery with curative intent for stage I NSCLC based on the 7th edition of the Union for International Cancer Control (UICC) TNM classification [Citation14]. Diagnosis of stage I NSCLC is generally based on positron emission tomography (PET) and/or computed tomography (CT) findings, sometimes confirmed by histological biopsy. Invasive mediastinal diagnostics such as endobronchial ultrasound (EBUS) can be used to exclude hilar or mediastinal lymph node metastases. The proportion of patients with stage I NSCLC receiving curative-intent surgery or radiotherapy was 91% and did not change over time (p = .12). The following patients were excluded from analysis: <18 years (n = 5); synchronous (n = 739) or metachronous tumors (n = 551); patients treated with chemotherapy or chemoradiotherapy (n = 119), neoadjuvant treatment before surgery (n = 26), other surgical or bronchoscopic interventions (e.g., endoluminal laser therapy, cryotherapy, etc.) (n = 38), or best supportive care (n = 739). The latter group mainly comprises patients who are unfit to undergo a curative treatment (e.g., due to comorbidity or poor performance status). After exclusion, 9630 patients were eligible for analysis.
Statistical analysis
Patients were stratified by age: 18–59, 60–69, 70–79, and ≥80 years. Parameters predictive of treatment choice were assessed by tabulations. Multivariable analysis of treatment choice was performed by multilevel logistic regression including age, gender, year of diagnosis, clinical T-stage, and hospital lung cancer surgery status. Since the impact of age and year of diagnosis may not be linear, these factors were included in the model as categorical variables. To account for clustering of patients within hospitals, hospital of diagnosis was included as a random-effect parameter. Impact of the parameters is reflected by odds ratios (OR) and 95% confidence intervals (95% CI). Overall survival (OS) was calculated from day of diagnosis until day of death or 1 February 2019 using actuarial analysis. Median follow-up for censored patients was 48 months. Median time between diagnosis and start of treatment was 30 days. Variation in survival in hospitals with and without lung cancer surgery was tested for significance by logrank testing. p Values <.05 were considered statistically significant. All analyses were performed in Stata V14 (College Station, TX, USA).
Results
Treatment of NSCLC and patient characteristics
9630 patients were identified, who were diagnosed in 77 hospitals. The number of patients receiving either surgery or radiotherapy for stage I NSCLC increased from 1660 in 2012 to 2198 in 2016 ().
Table 1. Parameters predictive of choice for surgery.
Surgery
There was only a small change in the total number of surgical resections per year (), but the proportion of patients receiving a surgical resection decreased with time. The number of patients being operated fluctuated between 1056 (2012) and 1137 (2015). The majority of surgical procedures were performed by video-assisted thoracoscopic surgery (VATS); this proportion increased from 57% in 2012 to 74% in 2016. Surgical resections included: lobectomy (84.7%), wedge resection (5.0%), bilobectomy (4.5%), pneumonectomy (3.6%), and segmentectomy (2.2%). Preoperative pathological confirmation of malignancy in cT1A, cT1B, and cT2A tumors was 38%, 56%, and 70%, respectively. Postoperatively, 23% of tumors were pathologically upstaged to stage II or higher. Two percent of patients with pathological stage I and 49% of patients with pathological stage II or higher received postoperative treatment (chemotherapy or chemoradiotherapy), which was 12.4% of operated patients.
Radiotherapy
The number of patients receiving radiotherapy increased from 604 (36%) in 2012 to 1095 (50%) in 2016. The use of SABR in patients receiving radiotherapy remained fairly constant: 90% in 2012 and 93% in 2016. There was pathological confirmation of malignancy in 49% of all patients receiving radiotherapy in this study period. The proportion of patients treated with radiotherapy increased with age, year of analysis, and decreasing tumor size ().
Treatment variation between hospitals
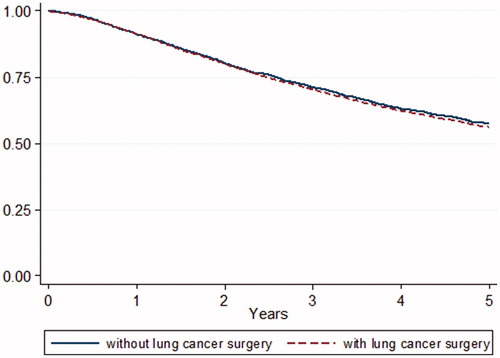
Of the 77 hospitals in total, lung cancer surgery was performed in 50 hospitals in 2012 and 43 in 2016. Hospital volume was not evaluated because this study only included stage I NSCLC. In the period 2012–2016, 59% (range 41–75%) of patients diagnosed with stage I NSCLC in hospitals with in-house lung cancer surgery received an operation, compared with 51% (range 18–71%) of patients diagnosed in hospitals without in-house lung cancer surgery (OR 1.25; 95% CI 1.01–1.54; p = .04). In this period, radiotherapy preference increased from 41% to 55% in hospitals without in-house surgery and from 36% to 48% in hospitals with in-house surgery. Five-year OS (for curative-intent surgery and radiotherapy combined) did not differ between hospitals with and without in-house lung cancer surgery (56% and 58%, respectively; p = .26) ().
Discussion
During the past decades, centralization of lung cancer surgery led to a reduction of hospitals with in-house lung cancer surgery in the Netherlands. Treatment patterns for patients with stage I NSCLC differed between hospitals with and hospitals without in-house lung cancer surgery. Resection rates were significantly higher for patients diagnosed in hospitals with in-house lung cancer surgery. In hospitals with in-house lung cancer surgery, between 41 and 75% of patients with stage I NSCLC were operated on. In hospitals without lung cancer surgery, between 18 and 71% of patients were referred for surgery. However, despite this variation, the combined OS for patients treated with curative-intent surgery or radiotherapy was not worse in hospitals without lung surgery facilities. This suggests that, by further centralization of lung cancer surgery, treatment outcome in Dutch hospitals may not be affected (worsened) for stage I NSCLC.
Of all patients with stage I NSCLC, the proportion receiving curative-intent surgery or radiotherapy remained constant during this period, while the proportion of patients operated on decreased and the use of radiotherapy increased. However, this change in pattern of care did not lead to detectable differences in the OS (for surgery and radiotherapy combined). Variation in outcome between individual hospitals was not assessed. The fact that this treatment variation occurred, despite a high level of cooperation and videoconferencing between Dutch hospitals [Citation12], indicates that more effort is needed to understand and minimize variation in decision-making. Although it cannot be concluded from the results presented here, it has previously been argued that minimizing treatment variation and standardizing treatment is expected to improve lung cancer outcomes [Citation15].
The pathological upstaging of resected tumors resulted in 12.4% of surgical patients receiving adjuvant therapy (which was not used after radiotherapy, since there is no upstaging). This is concordant with other publications from the Netherlands [Citation16]. Yet, the impact of the adjuvant therapy was not detectable in the survival curves comprising varying proportions of surgery and radiotherapy patients. With the development of new strategies for adjuvant treatment (e.g., immunotherapy), invasive staging becomes increasingly important [Citation17].
The main strength of this study is that it is population-based, including 9630 patients diagnosed in 77 different Dutch hospitals. However, limitations include the fact that information on comorbidity, performance status, and complications is not registered in the NCR and therefore not available for analysis. Therefore, residual variation in patient characteristics might exist, which cannot be adjusted for, and which could contribute to the differences in treatment strategies and the similarities in survival outcome. Also, information on cause of death was not available due to privacy restrictions, making it impossible to differentiate between cancer-specific survival and death from other causes. Therefore, possible differences in non-cancer related mortality between the surgery and radiotherapy group may bias our results.
In conclusion, centralization has led to a reduction in hospitals with in-house lung cancer surgery. Treatment of stage I NSCLC varied between hospitals with and without in-house lung cancer surgery. In this large population-based analysis, absence of lung surgery facilities in hospitals influenced treatment choice, but did not lead to worse OS. The relationship between centralization, treatment selection, and survival merits further investigation.
Disclosure statement
Dr. De Langen reports grants from AstraZeneca, grants from BMS, grants from MSD, non-financial support from Roche, non-financial support from Merck-Serono, grants from Boehringer, outside the submitted work. Dr. Dahele reports grants and personal fees from Varian Medical Systems, outside the submitted work.
References
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(4):1–21.
- Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline Summary. J Oncol Pract. 2018;14(3):180–186.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076.
- Nyman J, Hallqvist A, Lund JÅ, et al. SPACE – a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8.
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28(35):5153–5159.
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):348–353.
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93(5):989–996.
- Subramanian MP, Meyers BF. Surgical resection versus stereotactic body radiation therapy for stage I NSCLC: can randomized trials provide the solution? Cancers (Basel). 2018;10(9):310.
- Zhang B, Zhu F, Ma X, et al. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non-small cell lung cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;112(2):250–255.
- Wouters MW, Jansen-Landheer ML, van de Velde CJ. The quality of cancer care initiative in the Netherlands. Eur J Surg Oncol. 2010;36(1):3–13.
- Damhuis RA, Maat AP, Plaisier PW. Performance indicators for lung cancer surgery in the Netherlands. Eur J Cardiothorac Surg. 2015;47(5):897–903. 903–904.
- Ten Berge M, Beck N, Heineman DJ, et al. Dutch Lung Surgery Audit: a national audit comprising lung and thoracic surgery patients. Ann Thorac Surg. 2018;106(2):390–397.
- Von Meyenfeldt EM, Gooiker GA, van Gijn W, et al. The relationship between volume or surgeon specialty and outcome in the surgical treatment of lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2012;7(7):1170–1178.
- Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15(1):4–9.
- Rankin NM, McGregor D, Stone E, et al. Evidence-practice gaps in lung cancer: a scoping review. Eur J Cancer Care (Engl). 2018;27(2):e12588.
- Heineman DJ, Ten Berge MG, Daniels JM, et al. Clinical staging of stage I non-small cell lung cancer in the Netherlands – need for improvement in an era with expanding nonsurgical treatment options: data from the Dutch Lung Surgery Audit. Ann Thorac Surg. 2016;102(5):1615–1621.
- Owen D, Chaft JE. Immunotherapy in surgically resectable non-small cell lung cancer. J Thorac Dis. 2018;10(3):404–411.