Background
Death of colorectal cancer (CRC) is primarily associated to distant metastases, present at the time of diagnosis or appearing later following a cancer-free period. At time of diagnosis, 75% of patients present with non-metastatic disease, for which standard of care is curative surgery [Citation1]. Following curative surgery 15–20% of patients experience recurrence of which 70–90% are detected within 3 years of surgery [Citation2–9]. Due to the high recurrence risk, adjuvant chemotherapy is standard of care for high risk UICC stage II and UICC stage III patients [Citation10], but still ∼33% experience recurrence [Citation4,Citation6,Citation11]. Extending duration of adjuvant chemotherapy offers no further reduction in recurrence rates [Citation12–15]. Consequently, the only alternative option after adjuvant chemotherapy is surveillance aimed at detecting recurrence sufficiently early to allow efficient treatment [Citation16–18].
Current surveillance strategy in Denmark consists of computed tomography (CT) scans of thorax and abdomen at postoperative months 12 and 36, and colonoscopy every fifth year until age 75 years. Increasing intensity of CT based surveillance for stage II-III has not been found to improve survival [Citation5,Citation19,Citation20]. However, more patients receive curative intended surgery for recurrence with intense surveillance [Citation5].
Methods to identify patients with microscopic residual disease after adjuvant chemotherapy are highly needed [Citation21]. Minimally-invasive blood-based analysis of circulating tumor DNA (ctDNA) has this potential, since patients with positive ctDNA following adjuvant chemotherapy possess a risk of recurrence of close to 100%, and ctDNA negative patients a risk as low as 10% [Citation22–24]. Also, longitudinal ctDNA analysis detect relapse with an average lead time of ∼9 months compared to standard-of-care CT imaging [Citation22,Citation23].
Rationale
The hypothesis of this study is that ctDNA guided post-operative surveillance combining ctDNA analysis and radiological assessments will result in a higher fraction of patients with recurrent disease receiving intended curative or local metastasis-directed treatment as compared to current Danish surveillance strategy.
Aim and objectives
The aim of this study is to conduct a randomized controlled trial investigating the benefit of ctDNA guided postoperative surveillance for UICC stage II high-risk and UICC stage III CRC patients compared to the current standard-of-care CT scan surveillance. The main objective is to investigate if ctDNA guided surveillance increases the proportion of patients receiving curative intended resection or local metastasis directed treatment (Fraction receiving Curative Intervention, FCI). Secondary objectives include investigation of quality of life (QoL), overall survival, cost-effectiveness and time to recurrence detection.
Material and methods
Study design
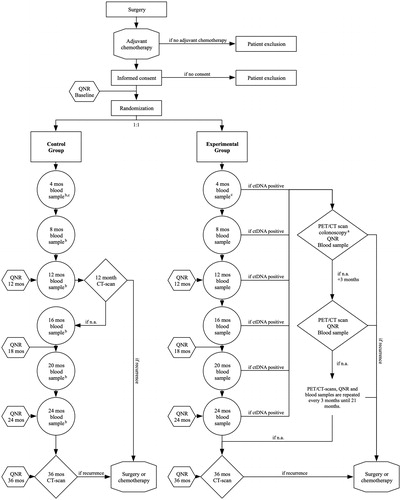
IMPROVE-IT2 is a randomized multicenter trial comparing the outcomes of ctDNA guided post-operative surveillance and standard-of-care CT scan surveillance. The trial design is a parallel group study with 1:1 allocation. The trial profile is illustrated as a flow chart in . The full protocol (see Supplementary file) is consistent with current Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines [Citation25,Citation26].
Figure 1. Trial profile. ctDNA: circulating tumor DNA; PET/CT: Flour-Deoxy-Glucose Positron Emissions Tomografi scan; FCRI: fear of cancer recurrence inventory; IES-C: Impact of Event Scale - Cancer; Mos: months; N.a.: nothing abnormal; QNR: questionnaires. aA colonoscopy is performed in case of ctDNA + blood sample and a PET-CT scan with no evidence or suspicion of residual disease or recurrence. bBlood samples from the control group are not analyzed until end of study, but serve to enable post-trial comparison of oncological outcomes for the two groups stratified for ctDNA-status. c4 month blood sample is taken in case of 3 months adjuvant chemotherapy regime. In case of 6 months adjuvant chemotherapy the 8 month blood sample will serve as the first blood sample. QNR: At baseline and months 12, 18, 24 and 36 months patients complete the QoL questionnaires including EORTC QLQ-C30, EQ-5D-5L, FCRI, and IES-C. Further, ctDNA positive patients will complete the QNR questionnaires before each PET-CT scan.
Study population and eligibility criteria
Currently, 11 of 17 surgical centers in Denmark are participating. In total, the trial will include 250 patients resected for CRC and treated with adjuvant chemotherapy. The following eligibility criteria will be applied:
Inclusion criteria:
Colon or rectal cancer, tumor stage III (pT1-4N1-2,cM0) or stage II high risk (pT4N0,cM0 and pT3N0, cM0 with either of the risk factors (<12 lymph nodes, anastomotic leakage, emergency surgery, signet ring adenocarcinoma) described in the national guideline for adjuvant therapy to stage II cancer)
Received intended curative resection and found eligible for adjuvant chemotherapy
Exclusion criteria:
Not treated with adjuvant chemotherapy
Synchronous colorectal and non-colorectal cancer diagnosed perioperatively (except skin cancer other than melanoma)
Other cancers (excluding CRC or skin cancer other than melanoma) within 3 years from screening
Recruitment
Patients who meet the eligibility criteria are approached at the treating surgical department. The patients are given written project information and oral information by a trained health care professional. As the project involves genomic sequencing, the participants are offered genetic counseling before obtaining written informed consent. Informed consent is given on a voluntary basis and has to be obtained prior to commence of any study-related procedure. The consent may be withdrawn at any time without having any impact on current or future treatment.
Randomization
Randomization is performed using a predefined randomization module in the Research Electronic Data Capture (REDCap) database [Citation27,Citation28]. A block randomization is set up to stratify by tumor location, pN-stage and standard-of-care surveillance program intensity, as these factors are associated with risk, location and prognosis of recurrence.
Interventions
The experimental group:
ctDNA analysis will be performed every 4 months postoperatively (4, 8, 12, 16, 20 and 24). At time of first positive ctDNA, patients undergo a whole-body FDG-PET/CT scan for radiological assessment. If the initial assessment is without evidence of recurrence or another cancer, patients will be offered a colonoscopy once and high-intensive radiological surveillance with FDG-PET/CT scans every 3 months, until recurrence detection or 21 months have passed.
The control group:
Patients will undergo surveillance according to current Danish Guidelines with CT scans at months 12 and 36 postoperatively. Longitudinal blood samples will be collected at same time-points as in the experimental group but not analyzed until end of trial.
Both groups complete QoL questionnaires at baseline (prior to randomization and start of surveillance), and at 12, 18, 24 and 36 months. The questionnaire includes European Organization for Research and Treatment of Cancer Quality of Life questionnaire, Core 30 (EORTC QLQ-C30 ver. 3.0) [Citation29], European Quality of Life – 5 Dimensions (EQ-5D-5L) [Citation30,Citation31], Fear of Cancer Recurrence Inventory (FCRI) [Citation32], and Impact of Events Scale for Cancer (IES-C) [Citation33]. Before every FDG-PET/CT scan in ctDNA positive patients, the patients also complete the questionnaires.
Trial status
Inclusion of patients is set to begin January 2020 and expected to end in December 2020. The primary endpoint will be reached and ready for publication in December 2023. The planned 5 years of follow-up will be complete and available for all patients by December 2025. The trial has been registered in the Clinical Trials database: NCT04084249.
ctDNA analysis
Circulating free DNA will be isolated from the longitudinally collected plasma samples using standard methods. DNA from the patients’ tumor will be whole exome sequenced to identify somatic mutations suitable for monitoring in blood plasma using droplet digital PCR (ddPCR). In cases where ddPCR is not possible, detection and quantification of ctDNA will be done using an optimized version of our recently developed ultra-sensitive targeted sequencing approach [Citation34]. The targets include the regions of the genome that are most frequently mutated in CRC ().
Table 1. Colorectal cancer (CRC) driver genes targeted by the CRC cfDNA sequencing panel.
Biobank
Residual blood and tissue biopsies as well as clinical and sequencing data will be transferred to the Colorectal Cancer Research Biobank at Aarhus University Hospital for future research. The research biobank is approved by the Danish Data Protection Agency (j. no. 1-16-02-27-10).
Ethical considerations
The risks and ethical concerns related to the project are limited. We will perform ultra-deep targeted sequencing of plasma DNA and leukocyte DNA to identify tumor specific alterations in the plasma DNA. The risk of accidentally finding a potential clinically relevant genomic variant related to inherited diseases is extremely low, and practically hypothetical.
Ionizing radiation is a known carcinogenic and increases the risk of cancer. ctDNA-positive patients in the experimental group receive intensified follow-up with 18FDG-PET combined with a diagnostic contrast-enhanced CT scan, 18FDG-PET/CT, resulting in a total radiation dose of app. 23.6 mSv pr. scan. We state that the potential benefit from identifying recurrence at an early stage with possible curative treatment, clearly outweighs the hypothetical risk (<3%) of a radiation-induced secondary cancer from participating in this study.
Definition of endpoint
The primary endpoint of this study is the fraction of patients with recurrence receiving intended curative or local metastasis-directed treatment (FCI) as defined and prospectively evaluated by a trial office appointed endpoint committee to ensure uniformity across study sites.
Secondary endpoints include:
Overall survival at 3 and 5 years (3-yr OS and 5-yr OS)
Time to clinical recurrence (TTCR)
Time to molecular recurrence (TTMR)
Quality of Life (QoL) by use of EORTC QLQ-C30, version 3.0
Fear of Cancer Recurrence Inventory (FCRI)
Impact of Events Scale Cancer (IES-C)
Cost-effectiveness (CE)
Data management
This study complies with the Data Protection Act and the General Data Protection Regulation (GDPR). The project has been reported to the Central Denmark Region.
Data will be entered into electronic case report forms (eCRF) established using the Research Electronic Data Capture (REDCap) framework [Citation27,Citation28] at Aarhus University. A quality control of data will be performed to ensure that data entry and verification have been performed correctly in accordance to pre-defined instructions.
Monitoring
The trial sites will be visited by the Clinical Trial Manager (Monitor) periodically at times agreed with the investigator. The monitor has to ascertain protocol compliance and that the trial conduction conforms to applicable regulatory requirements and established rules for Good Clinical Practice.
The monitor will review and verify the data collected in the eCRFs against the source documents and address any discrepancies found in the data. All corrections will be documented in an audit trail.
Statistical analysis and sample size
All data will be presented using descriptive statistics and analyzed as intention-to-treat.
Kaplan-Meier estimates will be used to estimate median times to recurrence, disease or death, stratified according follow-up intensity. Endpoints will be assessed using the log-rank test or a Cox regression model, with time to event as response variable and intensity of follow-up as a factor.
QoL and fear of recurrence analysis will be done using the validated Danish versions of the EORTC QLQ-C30 health-related QoL questionnaire[Citation29], the EQ-5D-5L [Citation30,Citation31], the FCRI [Citation32], and the IES-C [Citation33].
For cost-effectiveness analysis, QoL/Utility weights for the quality-adjusted life years parameter will be QLU-C10D based on EORTC QLQ-C30.
Sample size calculations are based on our experience and preliminary data from the IMPROVE trial (ClinicalTrials.gov Identifier: NCT03637686). In total, 250 patients will be included in IMPROVE-IT2 (125 in each group). The expected recurrence rate is ∼33% (n = 84), hence, with a 1:1 randomization, we expect 42 recurrences in each group. With standard-of-care surveillance, approximately 15% of recurrences are eligible for curative treatment. Assuming this fraction can be tripled, we will reach a power of 80% to detect a difference at a 5% significance level with 35 recurrence patients in each arm.
Dissemination
The results from IMPROVE-IT2 are planned for publication in peer-reviewed scientific journals.
Discussion
Metachronous metastases most frequently affect the liver and the lungs. If it is detected too late for curative resection, less than 10% of patients are alive after 5 years [Citation35–40]. The ability to resect and the survival after resection are negatively affected by increasing numbers of metastatic sites and the size of the metastases [Citation35,Citation36,Citation41]. With current surveillance, only ∼15% of recurrences are eligible for curative resection or local treatment [Citation42,Citation43]. Thus, early detection of recurrences when tumor burden is low is paramount, not only to increase the rate of curative resections, but also to improve survival following resection.
The minimal-invasive blood-based analysis of ctDNA is based on the principle that tumors shed DNA fragments with a half-life of less than two hours [Citation44] into the blood [Citation45]. Consequently, patients with residual disease are likely to be ctDNA positive during follow-up. The plasma level of ctDNA may initially be below the lower detection level of the analysis, but we have shown that ctDNA undergoes a 5-fold increase from first ctDNA detection to radiological relapse detection with standard surveillance [Citation46]. This underlines the importance of collecting blood samples longitudinally as the residual disease grows, resulting in ctDNA levels reaching a detectable level during the course of follow-up.
Longitudinal blood samples will be collected at the same time points in the control group as in the experimental group but not analyzed until end of trial. They serve to enable post-trial comparison of oncological outcomes for the two groups stratified for ctDNA-status. Besides the additional blood sampling, the control group undergo surveillance in complete accordance to national guidelines and, thus, represents current surveillance practice.
A potential negative effect of recurrence surveillance is increased fear of recurrence and reduced QoL. To enable comparison of the impact on QoL and fear of recurrence of the two surveillance programs, we will collect QoL and fear of recurrence information longitudinally during surveillance.
IMPROVE-IT2 is the first randomized controlled trial of its kind and has the potential to provide a tool for earlier detection of recurrent disease and to identify more patients eligible for curative treatment. IMPROVE-IT2 is a national study. Consequently, if the trial confirms that ctDNA-guided surveillance is superior to standard-of-care surveillance, and if the national health authorities decide to implement it, this can be done at a national level immediately.
Ethics approval and consent to participate
This study is approved by the Central Denmark Region Committees on Health Research Ethics (j. no. 1-10-72-162-19). All participants are offered genetic counseling before obtaining written informed consent. Informed consent is given on a voluntary basis and is obtained prior to commencement of any study-related procedure. The consent maybe withdrawn at any time without consequence. This study will not present any personal data in any way and will not require consent for publication by any participant(s).
Author contributions
JN drafted this manuscript. KAG, LHI, TJ, JS and CLA designed the study. TVH drafted the full protocol. All authors reviewed and approved the final manuscript.
| Abbreviations | ||
| CRC | = | colorectal cancer; |
| CT | = | computed tomography |
| ctDNA | = | circulating tumor DNA |
| FCI | = | fraction receiving curative or local metastasis Intervention; |
| QoL | = | quality of life |
| FCRI | = | fear of cancer recurrence inventory |
| IES-C | = | impact of events scale for cancer |
| TTCR | = | time to clinical recurrence |
| AR | = | adherence rate |
| TTMR | = | time to molecular recurrence; |
| ddPCR | = | droplet digital Polymerase Chain Reaction |
| 3yr-OS | = | 3 years overall survival |
| 5yr-OS | = | 5 years overall survival |
| CE | = | cost effectiveness |
| eCRF | = | electronic case report form |
Supplemental Material
Download Zip (656.5 KB)Acknowledgments
The authors want to thank the IMPROVE-study-group and all participating surgical departments for their corporation in this study.
Disclosure statement
The author(s) have no affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the study subject or materials discussed in the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Additional information
Funding
References
- Iversen LH, Green A, Ingeholm P, et al. Improved survival of colorectal cancer in Denmark during 2001–2012 – the efforts of several national initiatives. Acta Oncol. (Stockholm, Sweden). 2016;55(supp 2):10–23.
- Kobayashi H, Mochizuki H, Sugihara K, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141(1):67–75.
- Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire Swedish population. Dis Colon Rectum. 2018;61(9):1016–1025.
- Ikoma N, You YN, Bednarski BK, et al. Impact of recurrence and salvage surgery on survival after multidisciplinary treatment of rectal cancer. J Clin Oncol. 2017 ;35(23):2631–2638.
- Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311(3):263–270.
- Wieldraaijer T, Bruin P, Duineveld LAM, et al. Clinical pattern of recurrent disease during the follow-up of rectal carcinoma. Dig Surg. 2018;35(1):35–41.
- Duineveld LA, van Asselt KM, Bemelman WA, et al. Symptomatic and asymptomatic colon cancer recurrence: a multicenter cohort study. Ann Fam Med. 2016;14(3):215–220.
- Reece MM, Chapuis PH, Keshava A, et al. When does curatively treated colorectal cancer recur? An Australian perspective. ANZ J Surg. 2018;88(11):1163–1167.
- van Gestel YR, de Hingh IH, van Herk-Sukel MP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. 2014;38(4):448–454.
- Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23(10):2479–2516.
- Lash TL, Riis AH, Ostenfeld EB, et al. Associations of statin use with colorectal cancer recurrence and mortality in a Danish cohort. Am J Epidemiol. 2017;186(6):679–687.
- Sobrero A, Sciallero S. Adjuvant therapy for colon cancer: 12 months, 9 months, 6 months … why not 3 months?. Ann Oncol. 2005;16(4):521–522.
- Iveson TJ, Kerr RS, Saunders MP, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19(4):562–578. 562–578.
- Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177–1188.
- Des Guetz G, Uzzan B, Morere JF, et al. Duration of adjuvant chemotherapy for patients with non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2010;(1):CD007046.pub2.
- Wyrwicz L, Tiret E, Glynne-Jones R, on behalf of the ESMO Guidelines Committee, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–iv40.
- Nordlinger B, Beretta GD, Labianca R, on behalf of the ESMO Guidelines Working Group, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–72.
- Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713–725.
- Jeffery M, Hickey BE, Hider PN, et al. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:Cd002200.
- Wille-Jorgensen P, Syk I, Smedh K, et al. Effect of more vs less frequent follow-up testing on overall and colorectal cancer-specific mortality in patients with stage II or III colorectal cancer: the COLOFOL randomized clinical trial. JAMA. 2018;319(20):2095–2103.
- Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26(4):644–656.
- Reinert T, Scholer LV, Thomsen R, et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut. 2016;65(4):625–634.
- Scholer LV, Reinert T, Orntoft MW, et al. Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res. 2017 ;23(18):5437–5445.
- Tie J, Wang Y, Tomasetti C, et, al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92–346ra92.
- Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207.
- Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ (Clinical). 2013;346:e7586.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717–1727.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- Hovdenak Jakobsen I, Jeppesen MM, Simard S, et al. Initial validation of the Danish version of the Fear of Cancer Recurrence Inventory (FCRI) in colorectal cancer patients. J Cancer Surviv. 2018;12(6):723–732.
- Salsman JM, Schalet BD, Andrykowski MA, et al. The impact of events scale: a comparison of frequency versus severity approaches to measuring cancer-specific distress. Psycho-oncology. 2015;24(12):1738–1745.
- Phallen J, Sausen M, Adleff V, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med. 2017;9(403):eaan2415.
- Mitry E, Guiu B, Cosconea S, et al. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59(10):1383–1388.
- Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84(1):324–338.
- Boysen AK, Spindler KL, Hoyer M, et al. Metastasis directed therapy for liver and lung metastases from colorectal cancer-A population-based study. Int J Cancer. 2018;143(12):3218–3226.
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–259.
- Engstrand J, Nilsson H, Stromberg C, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18(1):78.
- Chakedis J, Schmidt CR. Surgical treatment of metastatic colorectal cancer. Surg Oncol Clin N Am. 2018;27(2):377–399.
- Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301.
- Elferink MA, de Jong KP, Klaase JM, et al. Metachronous metastases from colorectal cancer: a population-based study in North-East Netherlands. Int J Colorectal Dis. 2015;30(2):205–212.
- Snyder RA, for the Alliance for Clinical Trials in Oncology Network Cancer Surveillance Optimization Working Group, Hu CY, Cuddy A, et al. Association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. JAMA. 2018;319(20):2104–2115.
- Lo YM, Zhang J, Leung TN, et al. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet. 1999;64(1):218–224.
- Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24–224ra24.
- Reinert T, Henriksen TV, Christensen E, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124.
