Introduction
In 2018, the estimated number of new ovarian cancer cases worldwide was over 295,000, with almost 185,000 deaths, making it the eighth most common cancer in women in terms of incidence and mortality. Among gynecological cancers, it is the second leading cause of death worldwide [Citation1].
The cornerstones of epithelial ovarian cancer (EOC) treatment are surgery and chemotherapy. Operative treatment includes surgical staging and tumor debulking, while paclitaxel-carboplatin is the standard chemotherapy [Citation2]. The role of vascular endothelial growth factor (VEGF) and angiogenesis is important in EOC treatment. However, bevacizumab is the only anti-angiogenetic agent with a label for use in EOC [Citation3,Citation4]. Studies have found an approximately four-month benefit in progression-free survival (PFS) when bevacizumab is incorporated in the first-line treatment of advanced EOC or in a recurrent setting [Citation4–8]. A subgroup analysis of the first-line trial ICON7 showed that in high-risk patients, there was a significant improvement (nine months) in overall survival (OS) [Citation9]. In recurrent platinum sensitive setting, an OS benefit of 5 months was achieved in the GOG-0213 trial [Citation10]. In the Nordic countries, the use of bevacizumab in the first line treatment is generally restricted to patients with either stage IV, or suboptimally debulked stage III disease at a dose of 7.5 mg/kg Q3wk. In many hospitals, including ours, the high cost of bevacizumab generally allows it to be used only once in a patient’s treatment program, i.e., either in the primary or recurrent setting.
The aim of this study was to evaluate the true costs related to the non-surgical treatments of ovarian cancer from the first-line treatment to several later lines of therapy for up to five years of follow-up, with a special emphasis on the role of bevacizumab.
Material and methods
The cohort for this analysis consisted of patients who had received the diagnosis of EOC from 1 January 2011 through 31 December 2012 at Tampere University Hospital, Finland. The rationale of using this cohort was to create a follow-up of at least five years.
Patient information was collected retrospectively from the patient registry in 2017–2018. A structured form was used to collect the following data: date of diagnosis, operations concerning EOC, bevacizumab treatment (dates of drug administration and doses), chemotherapy (drugs, doses, dates of drug administration and number of treatment lines), other relevant medication which was used during and related to the EOC treatment, clinic visits (scheduled and emergency) and inpatient stays, relevant laboratory and imaging tests, and additional operations.
The cost calculations were performed based on the records from the internal management accounting systems (Ecomed ICS and Tableau), and using yearly information related to clinical expense data. Costs were calculated for each patient individually. Only costs concerning the Department of Obstetrics and Gynecology were taken into account. The costs of the primary operative treatment were not included since the objective was to analyze medical costs. Therefore, only costs during chemotherapy and/or bevacizumab treatment were calculated.
The chemotherapy medication costs include the work related to handling the medication and preparing the medication at the hospital pharmacy. Laboratory costs include the use of blood products.
We calculated the cost of granulocyte-colony stimulating factors (G-CSF) by using the Finnish market price during spring 2019 from Pharmaca Fennica as these were mainly used as prescription drugs.
If a patient participated in a clinical trial concerning EOC medication, those costs and medications were left out of the calculations and this ended her follow-up on our behalf. However, the possible occurrence of death was recorded.
The distributions of cost factors are shown by medians with interquartile ranges (IQRs) due to the skewed distributions and outliers (). Differences between categorical patient characteristics were tested by Pearson’s chi-square test or Fisher’s exact test. Due to the skew distributions, continuous patient factors were analyzed by the Mann–Whitney test. Analyses were performed using IBM SPSS Statistics for Windows (version 23.0, IBM Corp., Armonk, NY).
Table 1. Costs of the EOC treatment.
The study was approved by the Ethics Committee of Tampere University Hospital (identification code R17126). No informed consent was needed as there was no interference with the patient medication nor did it effect the patient’s treatment in any way. Individual patients cannot be identified from this report.
Results
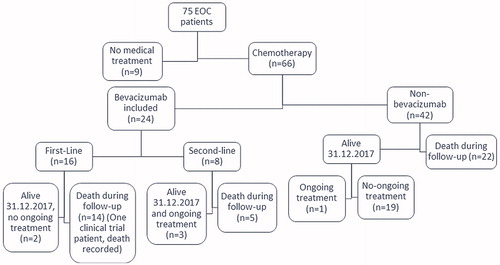
A total of 75 patients were diagnosed with EOC from 2011 through 2012 at Tampere University Hospital. Of them, 66 patients received chemotherapy, and their treatment costs form the focus of this analysis. Twenty-four patients (36%) received bevacizumab: 16 (67%) as a first-line therapy and 8 (33%) as a later line of therapy (). The mean age at diagnosis was 66.6 years, and most patients had an FIGO Stage III or IV disease at the time of the diagnosis, 30 and 17 patients, respectively. Almost 76% of patients needed hospitalization at some point during chemotherapy or bevacizumab treatment.
Most patients received 1–3 lines of chemotherapy, but up to six treatment lines were recorded from one patient. Only four patients had ongoing treatment at the end of the follow-up or at 31 December 2017 ().
The combined total costs of the medical EOC treatment including all variables for the entire cohort of 66 patients were 2,404,251 € of which the proportion of chemotherapy was 306,086 € or 13%. Of the total non-surgical costs for all patients, bevacizumab costs comprised 47.1%, which was the largest single expense even though it was administered to only 24 patients. In the bevacizumab group, the cost of the drug itself was 1,132,740 € or almost two-thirds or of all treatment costs. The median cost for ovarian cancer treatment/patient in the non-bevacizumab group was 7700 €, while in the bevacizumab group it was more than 10 times higher or 82,542 €. All variables presented higher costs in the bevacizumab group ().
Of the 24 bevacizumab patients, 16 received bevacizumab as a first-line treatment and eight as a second-line treatment. The median costs of the treatment/patient were 85,795 € and 76,311 €. In both groups, bevacizumab was the single largest expense (69% vs. 49%). Only one patient in the bevacizumab group was enrolled into a clinical trial during the follow-up. The costs prior to enrollment are included in the calculations.
Discussion
For the costs of all included patients, the cost of bevacizumab comprised almost half, or 47.1%. The relatively high contribution of the second largest cost or inpatient stay can be explained by the long follow-up during the course of the disease over consecutive recurrences and re-treatments, ending to progressive disease and death.
The bevacizumab group had higher median costs for all variables, which can partly be explained by the difference in the initial disease staging and the characteristics of a low-grade disease vs. a high-grade disease, as bevacizumab is used in patients with high-risk disease. Thus, the bevacizumab group more often had recurrence, resulting in more numerous lines of therapy. When the treatments stretched over a longer time period, the risk of complications and side-effects increased, and therefore the risk of emergency visits and hospitalization increased in parallel. The increased costs of imaging, laboratory and clinic visits are simply explained by the time consumed, i.e., the longer follow-up. When more treatment lines are used, the risk of neutropenia also increases, which explains the higher costs for G-CSF.
Neyt et al. made a cost-effectiveness analysis based on the material of four large trials on bevacizumab and ovarian cancer, GOG-0218, ICON7, OCEANS and AURELIA [Citation4–7,Citation11]. Compared to the present results, their calculations are in the same range. In the first line setting, Neyt et al. calculated based on GOG-0218 data that the mean costs related to bevacizumab are 44,286 €, while the actual costs were 52,766 € (Md 57,800€) in our study. The mean costs of bevacizumab treatment for recurrent disease in the present study are 36,060 € (Md 40,163 €), which is between the mean cost of bevacizumab in AURELIA and OCEANS trials, or 28,529 € and 53,591 €, respectively. If the chemotherapy visits are included in the calculations, the mean costs in our study and the OCEANS trial are quite similar or 45,991 €( Md 48,811 €) and 53,591 €, respectively.
The cost-effectiveness of bevacizumab has been questioned, as the main advantage of bevacizumab is a PFS benefit of approximately four months, but there is in general no OS benefit [Citation8]. As the cost-effectiveness is better in stage III and stage IV disease [Citation12], bevacizumab treatment should be prioritized in these patients [Citation13]. In ICON7, the median OS of high-risk patients treated with bevacizumab was 39.7 months, while it was 30.2 months in the control patients [Citation9]. Our median costs per patient for bevacizumab medication itself in first-line treatment was 57,800 €. If these costs are applied to the above OS figures, the incremental cost-effectiveness ratio (ICER) would be 73,010 €/quality adjusted life-year (QALY). This figure is low compared to the analysis by Neyt et al., who calculated an ICER of 157,816 € for GOG-0218 and 82,277 € for ICON7 in high-risk patients [Citation11]. The lower ICER in our calculations can partly be explained by the fact that some patients received only 2–4 doses of bevacizumab, which reduces costs significantly. In our first-line patients, there was also some diversity in doses (7.5 mg/kg n = 4, 15 mg/kg n = 12), which also contributes to our smaller bevacizumab costs compared to GOG-0218. This emphasizes the uniqueness of real-life calculations. If the threshold of reasonable QALY is considered to be 100,000 USD or 89,026 €, the role of bevacizumab at least in first-line treatment is supported. If our first-line costs were calculated with the dose of 7.5 mg/kg (which was proven to be a sufficient dose for first-line treatment in ICON7) by dividing the bevacizumab drug costs in half in patients who had received the dose of 15 mg/kg, we would reach an ICER of 44,763 €/QALY. This outcome makes the use of bevacizumab as the first-line treatment more acceptable from the health economic point of view.
Societal costs were not included in this study. As most of the patients were already retired, these costs were unlikely to have significant influence. We were neither able to reliably calculate the costs of treatment complications, as patients were not always treated at our department. However, it is well known that bevacizumab treatment increases the risk of gastrointestinal events, hypertension, proteinuria and thromboembolism [Citation8]. If all these costs were included in the analysis, the costs of bevacizumab treatment would be even higher. Anyway, the higher inpatient stay costs in the bevacizumab group can at least partially be explained by the complications related to its use.
The cohort of patients turned out to be very heterogeneous as the current guidelines for the use of bevacizumab in Finland were not established until 2012 [Citation14]. There was diversity in the treatment doses (first-line bevacizumab 7.5 mg/kg vs. 15 mg/kg), initiation and duration of treatment. If a later cohort had been chosen, the treatments would have been more homogenous, but the follow-up would have been shorter, and we wanted to calculate the costs over the entire course of the disease.
The major strength of this study is that the calculations are based on real-life situations rather than modeling costs to a specific drug administration protocol or guideline. As it was possible to obtain very accurate data from the internal management accounting system and the yearly expense data, the costs were processed in a very precise manner. Therefore, these calculations can be assumed to present a very accurate description of the chemotherapy and bevacizumab costs of EOC in a real-life setting, even though the cohort was rather small.
In conclusion, bevacizumab treatment was the single largest medical expense in EOC patients. For estimating future treatment costs, models can be helpful and accurate to a certain extent. However, real-life calculations provide a more accurate picture of all variables related to the treatment and show individual differences in the treatment.
Acknowledgments
We thank Dr Anssi Auvinen for giving valuable advice regarding this project.
Disclosure statement
Dr Vuorinen and MSc Luukkaala report no conflicts of interest. Dr Mäenpää has received honoraria from Roche, AstraZeneca, Tesaro, Clovis, Lilly, MSD and OrionPharma.
References
- WHO Statistics. Cancer Today, International Agency for Research on Cancer; [cited 2019 Apr 16]. Available from: http://gco.iarc.fr/today/
- Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374(9698):1371–1382.
- Gaitskell K, Martinek I, Bryant A, et al. Angiogenesis inhibitors for the treatment of ovarian cancer. Cochrane Database Syst Rev. 2014;9:CD007930.
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483.
- Perren TJ, Swart AM, Pfistere J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–2496.
- Pujade-Lauraine E, Hilpert E, Weber B, et al. Bevacizumab combined with chemotherapy for platinum resistant recurrent ovarian cancer: the AURELIA Open Label Randomized Phase III Trial. J Clin Oncol. 2014;32(13):1302–1308.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–2045.
- Ye Q, Chen H-L. Bevacizumab in the treatment of ovarian cancer: a meta-analysis from four phase III randomized controlled trials. Arch Gynecol Obstet. 2013;288(3):655–666.
- Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928–936.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(6):779–791.
- Neyt M, Vlayen J, Devriese S, et al. First- and second-line bevacizumab in ovarian cancer: a Belgian cost-utility analysis. PLoS ONE. 2018;13(4):e0195134.
- Barnett JC, Secord AA, Cohn DE, et al. Cost effectiveness of alternative strategies for incorporating bevacizumab into the primary treatment of ovarian cancer. Cancer. 2013;119(20):3653–3661.
- Randall L, Burger R, Nguyen H, et al. Outcome differences in patients with advanced epithelial ovarian, primary peritoneal and fallopian tube cancers treated with and without bevacizumab. Gynecol Oncol. 2013;130(1):e33–e34.
- Leminen A, Auranen A, Bützow R, et al. Ovarian cancer. Current care guidelines, The Finnish Medical Society Duodecim, 2012. Duodecim. 2012;128(12):1300–1301.