Abstract
Introduction: Population-based data on the implementation of guidelines for cancer patients in daily practice are scarce, while practice variation may influence patient outcomes. Therefore, we evaluated treatment patterns and associated variables in the systemic treatment of metastatic colorectal cancer (mCRC) in the Netherlands.
Material and methods: We selected a random sample of adult mCRC patients diagnosed from 2008 to 2015 from the National Cancer Registry in 20 (4 academic, 8 teaching and 8 regional) Dutch hospitals. We examined the influence of patient, demographic and tumour characteristics on the odds of being treated with systemic therapy according to the current guideline and assessed its association with survival.
Results: Our study population consisted of 2222 mCRC patients of whom 1307 patients received systemic therapy for mCRC. Practice variation was most obvious in the use of bevacizumab and anti-EGFR therapy in patients with (K)RAS wild-type tumours. Administration rates did not differ between hospital types but fluctuated between individual hospitals for bevacizumab (8–92%; p < .0001) and anti-EGFR therapy (10–75%; p = .05). Bevacizumab administration was inversely correlated to higher age (OR:0.2; 95%CI: 0.1–0.3) comorbidity (OR:0.6; 95%CI: 0.5–0.8) and the presence of metachronous metastases (OR:0.5; 95%CI: 0.3–0.7), but patient characteristics did not differ between hospitals with low or high bevacizumab administration rates. The hazard ratios for exposure to bevacizumab and anti-EGFR therapy were 0.8 (95%CI: 0.7–0.9) and 0.6 (95%CI: 0.5–0.8), respectively.
Discussion: We identified significant inter-hospital variation in targeted therapy administration for mCRC patients, which may affect outcome. Age and comorbidity were inversely correlated with non-administration of bevacizumab but did not explain inter-hospital practice variation. Our data suggest that practice variation is based on individual strategy of hospitals rather than guideline recommendations or patient-driven decisions. Individual hospital strategies are an additional factor that may explain the observed differences between real-life data and results obtained from clinical trials.
Introduction
Clinical guidelines are generated to facilitate the delivery of high-quality and evidence-based care, but population-based data on the implementation of guidelines in daily practice are scarce. Obviously, guidelines should leave room for personalisation of treatment to individual patients, but patient-independent practice variation is undesired since this may result in over- or undertreatment and thereby influence both patients’ quality of life and survival. Recent Dutch colorectal cancer guidelines (2008 and 2014) [Citation1] provide clear recommendations for the systemic treatment of patients with metastatic colorectal cancer (mCRC), but the adherence to these recommendations in daily practice has not been studied.
Improvements in median overall survival of mCRC patients have been achieved by the availability of more effective (targeted) drugs and more frequent use of resection of (mostly liver) metastases [Citation2]. The 2014 Dutch guideline recommendations for mCRC included the use of fluoropyrimidines (5-fluorouracil, capecitabine), oxaliplatin and irinotecan as cytotoxic drugs, and bevacizumab and cetuximab/panitumumab as targeted drugs [Citation1]. Data from retrospective analyses as well as prospective randomised trials suggest that the outcome of patients improves when all effective cytotoxic drugs are made available during the course of disease [Citation3–6]. Retrospective data suggest that the same principle applies for targeted drugs [Citation2].
However, especially the use of targeted drugs is accompanied with high costs for healthcare, which may affect prescription rates of these drugs [Citation7,Citation8]. In a recent Dutch multicentre study in first-line mCRC, in which the incidence of hand-foot syndrome was compared between two oral fluoropyrimidines, the use of bevacizumab was left to the discretion of the local physician [Citation9]. Approximately 40% of patients did not receive bevacizumab, which cannot fully be explained by medical contraindications. Furthermore, the use of salvage treatment with anti-EGFR therapy in this and another Dutch mCRC study [Citation10] was also much lower than expected. A regional study focussing on patients with metachronous metastases found suboptimal use of bevacizumab [Citation11]. Other international studies reported a wide range of chemotherapy and targeted therapy administration rates, but did not focus on patient outcomes [Citation12–17]. Population-based studies that examine practice variation in the systemic treatment of mCRC patients on hospital level, including co-variables that might influence therapy administration and the association of practice variation with survival, are lacking.
Therefore, the aim of our population-based study is to evaluate practice variation in the systemic treatment of mCRC between 2008 and 2015 in the Netherlands, to identify variables that are associated with practice variation and examine practice variation and its influence on overall survival.
Material and methods
Study design and population
We conducted a retrospective cohort study including mCRC patients diagnosed between 2008 and 2015 as registered in the Dutch National Cancer Registry (NCR). The Dutch NCR has nationwide coverage and includes all patients diagnosed with colorectal cancer and therefore guarantees a reliable reflection of the population. Our source population consisted of 106.998 stage I-IV CRC patients. The following data-items are routinely registered in the Dutch NCR up to February 2015: hospital, hospital type, period of diagnosis of metastases, gender, age, primary tumour localisation, metastatic sites at diagnosis, pathologic features (tumour stage, morphology and differentiation grade) and first-course treatment information including start and stop dates (surgery of the primary tumour, local treatment of metastases and first-line systemic treatment regimens). Since February 2015, additional variables including subsequent lines of systemic therapy are routinely collected.
In our study, we were interested in both first and subsequent lines of systemic therapy in the period between 2008 and 2015. For this purpose, an independent collaborator selected a representative random sample of approximately 4000 adult stage II-IV CRC patients from our source population (106.998 patients) specified for hospital type (4 academic, 8 teaching, 8 regional, randomly selected), tumour stage at diagnosis (II/III versus IV; ratio 1:1), histology (adenocarcinoma or adenocarcinoma-like tumours) and year of diagnosis.
Thereafter, we collected the following additional variables from patients’ electronic health records: comorbidity score (based on comorbid conditions in the following categories: pulmonary disease, cardiovascular disease, digestive disease, genitourinary disease, systemic and rheumatoid disease, neurologic disease, metabolic disease, coagulation disorders and infectious disease), molecular test results (BRAF, (K)RAS and mismatch repair status), registration of subsequent lines of systemic treatment including start and stop dates and treatment information in case of metachronous metastases. The indication for anti-EGFR therapy changed from KRAS wild-type tumours to RAS wild-type tumours during our study [Citation18,Citation19]. The Dutch National Cancer Registry does not differentiate between KRAS and NRAS mutated tumours.
Cohort classification
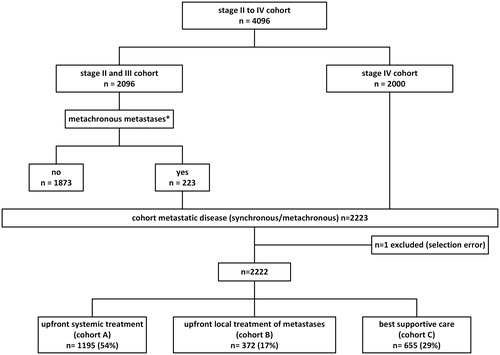
Our mCRC patient cohort consisted of patients with synchronous (stage IV) and metachronous metastases (extracted from patients with stage II/III disease). Metachronous metastases were defined as occurring ≥ 6 months after resection of the primary tumour. We divided our mCRC patient cohort into three subgroups: patients who received upfront systemic treatment (Cohort A), upfront local treatment of metastases (Cohort B), or best supportive care (Cohort C; ). Upfront local treatment of metastases consisted of surgical resection, HIPEC, radiofrequency ablation (RFA), microwave ablation (MWA) or (stereotactic) radiotherapy, either alone or in combination.
Definition of systemic treatment regimens
We defined lines of systemic treatment based on start and stop dates of individual chemotherapeutic and/or targeted agents and calculated the duration of each treatment regimen. In case a new agent was added to a regimen prior to the first radiological evaluation of response (usually after 8–9 weeks of treatment), we considered this agent as part of this treatment line. We considered an agent as reintroduction of therapy if it was administered after an interval of at least 3 months after previous administration. If this interval was less than 3 months, it was considered as continuation of an existing line of therapy. If reintroduction of an agent was preceded by a different line of treatment, reintroduction was considered as a new line of treatment. We defined maintenance treatment as continuation with a less intensive regimen upon achievement of at least stable disease (usually after 6 or 8 cycles of initial treatment). The duration of different treatment lines was determined by start and stop dates of treatment and was calculated based on the duration of initial treatment, maintenance treatment and reintroduction of therapies.
Guidelines recommendations
The most recent Dutch colorectal cancer guidelines [Citation1] (versions 2008 and 2014) recommend a fluoropyrimidine-containing schedule (monotherapy or combined with irinotecan or oxaliplatin) in combination with bevacizumab as standard of care in first-line treatment in both versions. Bevacizumab is not recommended in subsequent lines of systemic therapy. The 2014 version differentiates between patients with permanently unresectable and potentially resectable metastases. In case of the latter, first-line combination chemotherapy with bevacizumab is recommended in patients with (K)RAS mutant tumours. For patients with (K)RAS wild-type tumours, both bevacizumab and anti-EGFR antibodies are options in combination with chemotherapy. For patients with permanently unresectable metastases and (K)RAS wild-type tumours, anti-EGFR treatment is recommended as salvage treatment, either alone or in combination with chemotherapy. The Dutch guideline recommendations concerning systemic therapies were largely in line with other international mCRC guidelines at that time [Citation20,Citation21].
Outcomes
The primary outcomes are the frequencies and variety of systemic treatment regimens that are used for mCRC patients. We studied practice variation patterns among individual hospitals and between different types of hospitals and compared treatments with prevailing guideline recommendations (2008 until March 2014: 2008 guideline; as of April 2014: 2014 guideline). Secondary outcomes are the associations of demographic, clinical and tumour characteristics with (non)administration of systemic treatment and overall survival.
Statistical analysis
Descriptive statistics using frequency tables and percentages were generated for all study variables and are presented for cohort A, B and C separately. Variation in the administration of targeted therapies was assessed by χ2 tests or Fisher’s exact tests if applicable. Univariable logistic regression analysis was performed to determine the unadjusted association between demographic, clinical and tumour characteristics on the odds of being treated with bevacizumab and anti-EGFR therapy. Subsequently, we tested for multicollinearity between variables and the same variables were examined in multivariable logistic analyses to explore which variables were independently associated with targeted therapy treatment. Patients with known (K)RAS mutated tumours or with unknown (K)RAS mutation status were excluded from analyses that concerned anti-EGFR therapy. Overall survival (OS) was defined as the interval between date of diagnosis until date of death. Patients who were alive at the end of follow-up (1 February 2017) were censored. Crude survival rates were calculated with a Kaplan Meier method. We presented median OS in months with corresponding 95% confidence intervals (CI’s) and used a log-rank test to assess differences in survival curves between patient groups who were exposed versus not exposed to different systemic agents. A Cox proportional hazard ratio analysis was performed to study the influence of included co-variables on the risk of death. A co-variable was included in the analyses if we expected an association between the co-variable and practice variation and/or overall survival. All statistical analyses were performed using SAS, version 9.4. A p-value below .05 was considered as statistically significant.
Results
Study population
Our initial cohort consisted of 4096 patients (stage II/III: n = 2096; stage IV: 2000). In total, 223 stage II and stage III patients developed metastases during the follow-up of our study, resulting in a mCRC cohort of 2223 patients (). The low number of patients with metachronous metastases is due to a high percentage of included patients since 2015 with a relatively short follow-up time and because metachronous metastases of patients who do not receive treatment are not registered. One patient was excluded due to a selection error (squamous cell carcinoma instead of adenocarcinoma), resulting in a cohort of 2222 patients. The characteristics of included patients are presented in .
Table 1. Baseline characteristics of study population (cohort A, B and C).
Overview and sequence of systemic therapy in different patient cohorts
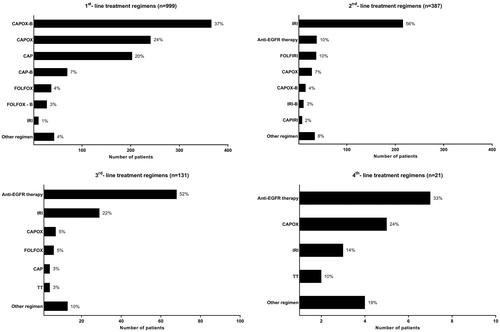
In total, 1307 out of 2222 mCRC patients (59%) from cohort A and cohort B, including patients with synchronous and metachronous metastases, were treated with systemic treatment for metastatic disease at some time point during the course of disease. The majority of Cohort A (n = 1195; upfront systemic treatment) received systemic treatment only (n = 999) without local treatment of metastases. The exposure to the different cytotoxic and targeted therapy agents of these 999 patients and an overview of the sequence of different lines of systemic therapy (including the number of patients who received maintenance treatment and/or reintroduction of treatment regimens) are presented in Supplementary Figures 1 and 2. The median duration of first-line treatment was 112 days, with CAPOX-B (capecitabine, oxaliplatin and bevacizumab; 37%), CAPOX (24%), capecitabine monotherapy (20%) and CAP-B (7%) as most commonly administered regimens (). In total, 387 patients (39%) received salvage systemic treatment, consisting in second-line most often of irinotecan monotherapy (56%), anti-EGFR therapy (10%) or 5-FU plus irinotecan (FOLFIRI; 10%; and Supplementary Figure 2). A minority of patients received third (n = 131; 13%), fourth (n = 21; 2%) and fifth (n = 1; <1%) line systemic treatment. Anti-EGFR therapy was predominantly prescribed in third and fourth line (52% and 33%, respectively; ).
Figure 2. Overview of first and subsequent lines of systemic treatment. Analysis is restricted to patients treated with systemic therapy only (n = 999). CAPOX: capecitabine & oxaliplatin; B: bevacizumab; CAP: capecitabine; FOLFOX: 5-fluorouracil & oxaliplatin; IRI: irinotecan; FOLFIRI: 5-fluorouracil & irinotecan; TT: Trifluridine tipiracil; Anti-EGFR therapy: cetuximab or panitumumab.
A minority of Cohort A patients (n = 196; 16%) underwent subsequent local treatment of metastases after upfront systemic treatment. These patients were predominantly treated with CAPOX (n = 84; 43%) and CAPOX-bevacizumab (n = 81; 41%) in first line before local treatment of metastases (Supplementary Figure 3). Three patients were treated with anti-EGFR therapy in first line.
A minority of Cohort B patients (112 out of 372 patients (30%)) received systemic therapy targeting metastases later in the course of disease after local treatment of metastases. An overview of systemic therapy lines is presented in Supplementary Figure 3.
Practice variation in targeted therapy administration
In total, 796 of 1307 patients (61%) who received systemic therapy, were exposed to targeted drugs. Tumours of 47% of patients (n = 610) were tested for (K)RAS mutations ((K)RAS wild-type: 301 (49%); (K)RAS mutant: 309 (51%)). Of systemically treated patients (n = 1307), 55% (n = 720) received bevacizumab and 13% of patients (n = 164) received anti-EGFR therapy during the course of disease. Specified for first-line regimens, 615 out of 1307 patients (47%) received bevacizumab and 10 out of 1307 patients (1%) received anti-EGFR therapy in first line. Bevacizumab administration did not differ between (K)RAS wild-type (n = 175) and (K)RAS mutant (n = 188) patients (p = .50). Of patients receiving anti-EGFR therapy, 134 patients (82%) were (K)RAS wild-type, 3 patients (2%) were (K)RAS mutant and 27 patients (16%) had an unknown (K)RAS status. If specified for proven (K)RAS wild-type patients (n = 301), 45% of patients (n = 134) received anti-EGFR therapy. Overall, 72 (K)RAS wild-type patients (24%) received treatment with subsequent bevacizumab and anti-EGFR therapy.
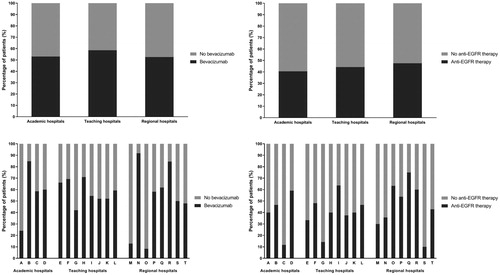
There was significant variation in the use of bevacizumab (p < .0001) and anti-EGFR therapy (p = .05) between individual hospitals, but not between different types of hospital (bevacizumab: p = .12; anti-EGFR therapy: p = .64). Bevacizumab administration between hospitals ranged from 8% to 92% (median: 58%; IQR: 49–68%) and anti-EGFR administration (in K(RAS) wild-type patients) from 10% to 75% (median: 41%; IQR: 34–58%; ).
Figure 3. Practice variation in bevacizumab and anti-EGFR therapy administration during the treatment of metastatic colorectal cancer. n = 1307. Analysis concerning anti-EGFR therapy is restricted to (K)RAS wild-type patients; n = 301.
More recent period of diagnosis (of metastases; 2011–2013 and 2014–2017 compared to 2008–2010), a higher age (>79 years compared to <60 years), comorbidity (≥ 2 versus no comorbidity) and metachronous metastases were associated with non-administration of bevacizumab (). All variables remained significantly associated with non-administration of bevacizumab after adjusting for all co-variables as listed in : period of diagnosis (OR: 0.5; 95% CI: 0.4–0.7 and OR: 0.7; 95% CI: 0.5–0.9), higher age (OR: 0.2; 95% CI: 0.1–0.3), comorbidity (OR: 0.6; 95% CI: 0.5–0.8) and metachronous metastases (OR 0.5; 95% CI: 0.3–0.7). Patients who received bevacizumab were significantly younger (mean = 63.9 (95% CI: 63.1–64.6); sd = 9.8) compared to patients who did not received bevacizumab (mean = 66.6 (95% CI: 65.7–67.5); sd = 11.0; p < .0001), but patients in hospitals with lower bevacizumab use were not significantly older. Patients not treated with bevacizumab were more likely to have hypertension (p < .0001), heart disease (p = .02), vascular disease (p = .05) thrombotic disease (p = .002) and/or renal disease (p = .01). However, we did not observe significantly higher incidence rates of these comorbidities in hospitals with lower bevacizumab use. Comorbidity rates were similar for (K)RAS wild-type patients who did or did not receive anti-EGFR therapy. There were 195 patients (15%) and 59 patients (20% in (K)RAS wild-type cohort) without comorbidities who were not exposed to bevacizumab and anti-EGFR therapy, respectively. There were no variables associated with the administration of anti-EGFR therapy in both univariable and multivariable analyses ().
Table 2. Unadjusted and adjusted odds ratios (OR) with 95% confidence intervals (95% CI) on bevacizumab and anti-EGFR therapy administration.
Survival analysis
The median overall survival (OS) of our mCRC cohort (n = 2222) was 14.3 months (95% CI: 13.4–15.0 months) with a 1-year survival rate of 56%. The median overall survival (OS) of Cohort C patients (best supportive care; n = 655) was 3.6 months (95% CI: 3.2–4.5 months). Patients in cohort A (upfront systemic therapy) who were subsequently treated with local treatment of metastases had a median survival of 36.1 months (95% CI: 30.8–39.7 months) compared to 14.0 months (95% CI: 13.1–14.8 months) for patients treated with systemic therapy only (p < .0001). Patients who were treated with systemic therapy only and were exposed to bevacizumab had a median OS of 15.9 months (95% CI: 14.6–17.0 months) compared to 12.2 months (95% CI: 10.9–13.1 months) for patients not exposed to bevacizumab (p = .002). Patients with a (K)RAS wild-type status (n = 238) treated with anti-EGFR therapy had a median OS of 23.8 months (95% CI: 20.0–27.2 months) compared to 14.9 months (95% CI: 13.1–16.5 months) for patients not treated with anti-EGFR therapy (p < .0001). Exposure to bevacizumab (HR: 0.8; 95% CI: 0.7–0.9) and exposure to anti-EGFR therapy (HR: 0.6; 95% CI: 0.5–0.8) remained associated with better survival after adjusting for all co-variables (Supplementary Table 1). A multivariable analysis on the effect of anti-EGFR therapy administration in patients with (K)RAS wild-type tumours (n = 238) confirmed this finding (HR: 0.6; 95% CI: 0.4–0.8).
Discussion
The results of our population-based longitudinal cohort study demonstrate substantial inter-hospital practice variation in the systemic treatment of mCRC patients between 2008 and 2015 in the Netherlands. The majority of mCRC patients (59%) received systemic treatment. Of systemically treated patients, 61% received targeted drugs during the course of their disease, which concerned bevacizumab in 55% of patients and anti-EGFR therapy in 45% of patients with K(RAS) wild-type tumours.
We observed significant inter-hospital variation in the use of targeted drugs, irrespective of hospital type. Absolute contraindications for anti-EGFR and bevacizumab treatment are rare, and for bevacizumab are limited to unhealed surgical wounds, major bleedings, recent haemoptysis, gastrointestinal perforation, uncontrolled hypertension and arterial thromboembolism [Citation22]. We found that patients who were not exposed to bevacizumab more often had a diagnosis of hypertension, heart disease, thrombotic disease or renal disease. However, the incidence of these comorbidities did not differ between patients from hospitals with low versus high bevacizumab use. Therefore, the large inter-hospital variation for targeted drugs administration in our study (range bevacizumab: 8% to 92% and anti-EGFR therapy: 10% to 75%) is not explained by the medical condition of patients. Unawareness of guideline recommendations is unlikely, given the laborious and adversarial procedure of the establishment of oncological guidelines. The significant and wide inter-hospital variation in the use of targeted drugs rather suggests a difference in hospital policy towards the use of expensive drugs in palliative setting, leading to guideline nonadherence and undesired practice variation. Although the use of bevacizumab and anti-EGFR antibodies in mCRC patients is covered by health insurance, their reimbursement in the Netherlands is part of a lump sum agreement between third party payers and hospitals. This allows hospitals to make individual strategic choices in the spending of their budget. However, these choices are usually not made public.
The survival rates of patients who were treated with systemic therapy in our study are lower compared to data from clinical trials. This is most likely attributable to the population-based design of our study compared to clinical trials, which concern selected patient populations under strict follow-up [Citation23,Citation24]. The median OS of patients treated with bevacizumab in our study is slightly lower compared to data from other observational studies in mCRC [Citation25–27]. This may be explained by differences in patient characteristics, since other studies restricted the inclusion to patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, a life expectancy of at least 3 months, and adequate organ function [Citation26], or (mostly) to patients who received combination chemotherapy in first line [Citation25,Citation27]. Our results reflect unselected real-life data of clinical practice.
The survival benefit that we observed for patients receiving bevacizumab and anti-EGFR therapy should be interpreted with caution, because of the influence of confounding by indication due to the observational design of our study. However, the effect of the addition of targeted therapy remained significant after adjusting for all co-variables listed with a reduced hazard of death if patients were exposed to bevacizumab or anti-EGFR therapy. Therefore, our data support the survival benefit of targeted therapies in patients with mCRC as demonstrated in earlier studies.
We observed a significant association of period of diagnosis (2011–2013 and 2014–2017 compared to 2008–2009), higher age (>79 years compared to <60), comorbidity (≥2 versus 0) and the presence of metachronous metastases with non-administration of bevacizumab. The decrease in bevacizumab use over the years is remarkable, in which the 2008 publication of the less favourable results of the NO16966 study [Citation28] compared to the initial study by Hurwitz et al. of 2004 [Citation29] may have played a role. However, these data did not change the recommendation of bevacizumab as part of first-line treatment in the 2014 Dutch guideline. Our finding that bevacizumab administration rates are inversely correlated to higher age and comorbidity cannot be fully explained by evidence-based considerations about the applicability of this agent under such circumstances, since the use of bevacizumab in combination with chemotherapy has been shown safe and effective in elderly patients [Citation30,Citation31].
In conclusion, our results demonstrate undesired practice variation of guideline-recommended systemic treatment of mCRC patients in the Netherlands, which appears to affect clinical outcome of patients. Our data warrant continuous monitoring in daily practice of the implementation of up-to-date guideline recommendations, with documentation of reason(s) for guideline non-adherence, including possible financial barriers on the use of expensive drugs. This will provide valuable and currently unavailable information on the quality of oncological care, which will be highly relevant for the implementation in clinical practice of the increasing number of novel and often expensive cancer drugs. Observational population-based studies, such as currently ongoing in the Netherlands [Citation32], are highly suitable for this purpose. Lastly, our data show that the implementation of approved and guideline-recommended drugs into daily practice is not self-evident, and that strategies of individual hospitals add to the observed differences between real-life data and results obtained from clinical trials.
Supplemental Material
Download Zip (1.1 MB)Acknowledgments
The authors would like to thank the data-managers and data-collectors of the Netherlands Comprehensive Cancer Organisation (IKNL).
Disclosure statement
L. Keikes declares that she has no conflict of interest. M. Koopman has acted in advisory boards for Amgen, Bayer, Merck-Serono, Servier, and Roche; and has received unrestricted research funding from Merck-Serono and Roche. M.M. Stuiver declares that he has no conflict of interest. V.E.P.P. Lemmens has received unrestricted research funding from Roche. M.G.H. van Oijen has received unrestricted research funding from Amgen, Bayer, Lilly, Merck-Serono, Nordic and Roche. C.J.A. Punt has acted in advisory boards for Nordic Pharma and Servier.
Additional information
Funding
References
- Dutch colorectal cancer guideline. 2014. [cited 2020 Jan 28]. Available from: http://www.oncoline.nl/colorectaalcarcinoom
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. JCO. 2009;27(22):3677–3683.
- Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. JCO. 2005;23(36):9441–9442.
- Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007;370(9582):135–142.
- Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007;370(9582):143–152.
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. JCO. 2005;23(22):4866–4875.
- Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. JCO. 2015;33(10):1112–1118.
- Franken MD, van Rooijen EM, May AM, et al. Cost-effectiveness of capecitabine and bevacizumab maintenance treatment after first-line induction treatment in metastatic colorectal cancer. Eur J Cancer. 2017;75:204–212.
- Kwakman JJM, Simkens LHJ, van Rooijen JM, et al. Randomized phase III trial of S-1 versus capecitabine in the first-line treatment of metastatic colorectal cancer: SALTO study by the Dutch Colorectal Cancer Group. Ann Oncol. 2017;28(6):1288–1293.
- Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843–1852.
- Razenberg LG, van Gestel YR, de Hingh IH, et al. Bevacizumab for metachronous metastatic colorectal cancer: a reflection of community based practice. BMC Cancer. 2016;16(1):110.
- Song X, Zhao Z, Barber B, et al. Treatment patterns and metastasectomy among mCRC patients receiving chemotherapy and biologics. Curr Med Res Opin. 2011;27(1):123–130.
- Zhao Z, Pelletier E, Barber B, et al. Patterns of treatment with chemotherapy and monoclonal antibodies for metastatic colorectal cancer in Western Europe. Curr Med Res Opin. 2012;28(2):221–229.
- Hess GP, Wang PF, Quach D, et al. Systemic therapy for metastatic colorectal cancer: patterns of chemotherapy and biologic therapy use in US medical oncology practice. JOP. 2010;6(6):301–307.
- McLean J, Rho YS, Kuruba G, et al. Clinical practice patterns in chemotherapeutic treatment regimens for metastatic colorectal cancer. Clin Colorectal Cancer. 2016;15(2):135–140.
- Abrams TA, Meyer G, Schrag D, et al. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106(2):djt371.
- Zafar SY, Marcello JE, Wheeler JL, et al. Longitudinal patterns of chemotherapy use in metastatic colorectal cancer. JOP. 2009;5(5):228–233.
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. JCO. 2008;26(10):1626–1634.
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–1034.
- Benson AB III, Arnoletti JP, Bekaii-Saab T, et al. Colon cancer. J Natl Compr Canc Netw. 2011;9(11):1238–1290.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Hershman DL, Wright JD, Lim E, et al. Contraindicated use of bevacizumab and toxicity in elderly patients with cancer. JCO. 2013;31(28):3592–3599.
- Rothwell PM. External validity of randomised controlled trials: to whom do the results of this trial apply? Lancet. 2005;365(9453):82–93.
- Mol L, Koopman M, van Gils CW, et al. Comparison of treatment outcome in metastatic colorectal cancer patients included in a clinical trial versus daily practice in The Netherlands. Acta Oncol. 2013;52(5):950–955.
- Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist. 2009;14(9):862–870.
- Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847.
- Meyerhardt JA, Li L, Sanoff HK, et al. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. JCO. 2012;30(6):608–615.
- Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. JCO. 2008;26(12):2013–2019.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342.
- Aparicio T, Bouche O, Taieb J, et al. Bevacizumab + chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol. 2018;29(1):133–138.
- Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(11):1077–1085.
- Coebergh van den Braak RRJ, van Rijssen LB, van Kleef JJ, et al. Nationwide comprehensive gastro-intestinal cancer cohorts: the 3P initiative. Acta Oncol. 2018;57(2):195–202.