Introduction
Standard treatment in the curative setting of head and neck squamous cell carcinoma (HNSCC) includes radiotherapy, with or without surgery, and/or combined chemotherapy [Citation1–5]. Irrespectively of treatment intensity, the patients suffer from multiple acute side effects including pain, dysphagia, mucositis and xerostomia [Citation6–9]. This negatively affects the patients’ quality of life during radiotherapy [Citation10,Citation11]. The standard approach for assessing side effects are observer-based scoring systems [Citation12–14]. It has been shown in Danish and US head and neck cancer populations, that there is a discrepancy between observer-based scoring systems and the patient’s subjective assessment [Citation15,Citation16]. Patient Reported Outcome (PRO) can be used as a tool to obtain the patients’ subjective symptoms with a systematic approach [Citation17]. There is an increased focus on using PROs in head and neck cancer trials [Citation18,Citation19]. PRO can be used proactively, meaning that the clinicians actively reviews the patients’ PRO answers during therapy, and uses the feedback from patients to optimize the treatment e.g. management of side effects. Basch et al. have shown that patients with metastatic cancer receiving chemotherapy and proactive PRO counseling had improved Health-Related Quality of Life (HRQOL) [Citation20]. In the radiotherapy setting, proactive PROs has the potential to become a relevant tool to detect acute side effects in order to improve management and avoid patient deterioration. This is it yet to be shown in clinical trials.
In Denmark all centers treating HNSCC are part of the multidisciplinary Danish Head and Neck Cancer Group (DAHANCA). The healthcare system is government-funded for all citizens. This means that the study population represents all patients despite socioeconomic background [Citation21].
This paper describes the organization and methods behind the national DAHANCA 38 trial comparing systematic use of PRO during radiotherapy for HNSCC with standard clinical counseling. The hypothesis is that proactive use of PRO during radiotherapy will lead to improvements in the patients’ quality of life, based on more precise monitoring and management of side effects.
Material and methods
Patients
Inclusion criteria are age ≥18 years, a diagnosis of HNSCC, primary or post-operative curative radiotherapy with or without concomitant cisplatin, able to read and speak Danish, no serious cognitive deficits, and signed informed consent to participate in the study. Exclusion criteria is prior radiotherapy in the head and neck area, and proton therapy.
Design
The trial is designed as a prospective nation-wide, sequential cohort study, clinicaltrials.gov ID No. NCT03918382, DAHANCA 38 protocol version 1.3, 2nd July 2019 [Citation22], SPIRIT checklist, Supplementary material 1.
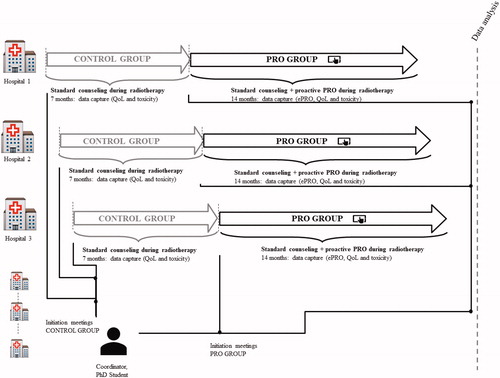
The study will be performed in a public health care system including all six centers treating HNSCC. Enrolled patients will receive radiotherapy ± cisplatin according to DAHANCA guidelines [Citation23]. The intervention in the trial is proactive use of PRO. The sequential design consists of two phases, see . In the first phase, the patients will be enrolled into the control group. The control group will receive standard clinical counseling according to current procedure. The second phase will be initiated when the last patient in the control group at the specific center has finished radiotherapy. In the second phase (the PRO group), the patients will receive standard clinical counseling plus proactive use of electronic PRO (ePRO) until 2 months after completion of radiotherapy, . Centers will be continuously accruing for 4 months starting in June 17th, 2019 at center 1. Each center has committed to include eligible patients and are responsible identification log. A screening log will be held of eligible patients.
Table 1. Overview of DAHANCA 38 assessment times, measures and tasks.
Electronic PRO questionnaire
We have previously shown that four validated head and neck cancer-specific PRO questionnaires are equally preferred by the patients [Citation24,Citation25]. Based on these results and the recommendations from Chera et al., the ePRO questions consist of 33 head and neck relevant items from the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™) library, 7 head and neck specific items from the European Organization for Research and Treatment of Cancer Quality (EORTC) item library and free text fields for additional symptoms, Supplementary material 2 [Citation18,Citation26]. The generic clinical PRO system, AmbuFlex, will be used for collection of ePROs. AmbuFlex has been used for research purposes in clinical epidemiological studies since 2004 [Citation27].
Trial flow
The patients have scheduled out-patient visits weekly during radiotherapy, two weeks and two months after completion of radiotherapy. At the scheduled out-patient visits, the patients are seen by a doctor and/or a nurse (standard clinical counseling). The number of visits depends on the individual patient´s treatment plan but is in general between 8-10 times from baseline until two months after completion of radiotherapy. The patients will go off study two months after radiotherapy and continue standard follow-up, .
In the standard consultation, the patients’ side effects are discussed and graded (DAHANCA toxicity score) [Citation14] by the clinician, and a focused physical examination is performed. The toxicity score and objective findings are reported to the DAHANCA database. The DAHANCA database contains information about tumour-related conditions, treatment, and demographic patient data.
All questionnaires in the trial are in Danish. At baseline (before start of radiotherapy), all participants complete a paper questionnaire about their level of education, their current work situation and their living situation. The EORTC QLQ-C30 [Citation28] and EuroQol Group’s five-level EuroQol five-dimensional questionnaire (EQ-5D-5L) [Citation29] will be completed by both groups at baseline, week four of treatment, at completion of radiotherapy and two months after completion of radiotherapy. These two PRO questionnaires will be used as passive collection of PRO in relation to our predefined outcomes and are completed on paper.
A case report form will be completed by the clinician after each scheduled out-patient visit to ensure endpoint data are captured.
Patients in the PRO group will complete the ePRO at baseline, weekly during radiotherapy until end of radiotherapy and two weeks and two months after radiotherapy completion. The patients will complete the ePRO on a tablet available in the waiting room before their consultation or at home. It is mandatory for the clinicians to review the ePRO answers prior to or during the consultation.
Patients in the PRO group will receive a Patient Reported Experience Measure (PREM), which is a feedback form to evaluate patient satisfaction with the ePROs [Citation30,Citation31].
Endpoints
Clinical data from all patients will be collected and will include age, stage, treatment regime, information on hospital admissions, treatment adjustments, and opioid treatment. The statistical analysis will include descriptive statistics of the two groups regarding number of enrolled patients, age, stage, treatment regime. A statistical analysis plan is available for details on planned data acquisition and analysis [Citation32]. Data will be captured in RedCap. The primary endpoint is difference in global HRQOL at the end of radiotherapy using the EORTC QLQ-C30 tool. Secondary and explorative endpoints are DAHANCA toxicity score [Citation14], time to start opioid treatment, time to tube-feeding/other feeding, weight loss, compliance to treatment (radiotherapy, chemotherapy, nimorazole), hospitalization due to toxicity except tube-feeding, quality-adjusted life years (QALYs), and PREM.
DAHANCA toxicity score is an objective grading of the patient’s symptoms by the clinician. It employs a categorical scale 0-4 in which 0 is no/nothing and 4 is severe. Differences between DAHANCA toxicity score in the PRO group and ePRO scores will be reported. Differences in time to opioid treatment, time to time to tube-feeding/other feeding, weight loss, number of hospitalizations reported and number of patients completing planned treatment between the control group and the PRO group will be reported. The EQ-5D-5L will be used to measure QALYs. The PREM questionnaire at end of radiotherapy covers the patient’s experience of the toxicity reporting, usefulness of the electronic PRO tool to cover symptoms and the patient’s experience of the communication with doctor/nurses and handling of the patient’s side effects [Citation31]. Descriptive statistics of QLQ-C30 domains will be performed.
Missing data will be handled according to EORTCs recommendations [Citation33]. We will investigate if data is missing at random and whenever possible cross-reference with national registries to acquire insights toward missing data.
Sample size calculation
The sample size calculation is based on the primary research question: difference in HRQOL at end of radiotherapy by EORTC QLQ-C30 (scale range 0-100, a higher score indicating better quality of life). We assume that the response is normally distributed with a standard deviation of 20 [Citation10,Citation11]. There will be two patients in the PRO group per patient in the control group. If the true difference in the PRO group and the control group means is 7, we will need 194 patients in the PRO group and 97 patients in the control group to yield 80% power to detect this assumed difference, using a two-sided test and a significance level of 0.05. Centers enrolling less than five patients in the control group will be excluded from the analysis. The sample size was determined from PS software (http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize).
Conclusion
This is the first national prospective study investigating proactive use of PRO during radiotherapy in a head and neck cancer population. The study will generate unique detailed information on quality of life, the patient´s perception of frequencies, severity and the development over time of acute side effects from radiotherapy. The study will explore if a systematic approach to the patients’ experience of symptoms will lead to optimized handling of side effects and better quality of life emphasizing the value of a more personalized care.
Ethical approval
The patients will receive verbal and written information and must give written informed consent which they can withdraw at any time.
The study will follow General Data Protection Regulation (GDPR) and is registered at the Capital Region of Denmark (ID. P-2019-24) and the Central Denmark Region (ID. 1-16-02-175-19). In a Danish setting the project does not need approval from the National Committee on Health Research.
Supplemental Material
Download Zip (57.5 KB)Disclosure statement
Varian Medical Systems has funded part of the salary for CHM, but do not have influence on the scientific work.
Additional information
Funding
References
- Rades D, Fehlauer F, Sheikh-Sarraf M, et al. Toxicity of two cisplatin-based radiochemotherapy regimens for the treatment of patients with stage III/IV head and neck cancer. Head Neck. 2008;30(2):235–241.
- Ho KF, Swindell R, Brammer CV. Dose intensity comparison between weekly and 3-weekly Cisplatin delivered concurrently with radical radiotherapy for head and neck cancer: a retrospective comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncologica (Stockholm, Sweden). 2008;47(8):1513–1518.
- Pignon JP, Le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14.
- Baujat B, Bourhis J, Blanchard P, et al. Hyperfractionated or accelerated radiotherapy for head and neck cancer. Cochrane Database Syst Rev. 2010;8(12):Cd002026.
- Lacas B, Bourhis J, Overgaard J, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18(9):1221–1237.
- Denham JW, Peters LJ, Johansen J, et al. Do acute mucosal reactions lead to consequential late reactions in patients with head and neck cancer? Radiother Oncol. 1999;52(2):157–164.
- Bentzen SM, Rosenthal DI, Weymuller EA, et al. Increasing toxicity in nonoperative head and neck cancer treatment: investigations and interventions. Int J Radiat Oncol Biol Phys. 2007;69(2):S79–S82..
- Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. JCO. 2008;26(21):3582–3589.
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–136.
- Loorents V, Rosell J, Salgado Willner H, et al. Health-related quality of life up to 1 year after radiotherapy in patients with head and neck cancer (HNC. ). SpringerPlus. 2016;5(1):669.
- Nyqvist J, Fransson P, Laurell G, et al. Differences in health related quality of life in the randomised ARTSCAN study; accelerated vs. conventional radiotherapy for head and neck cancer. A five year follow up. Radiother Oncol. 2016;118(2):335–341.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346.
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181.
- Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet (London, England). 2003;362(9388):933–940.
- Jensen K, Bonde Jensen A, Grau C. The relationship between observer-based toxicity scoring and patient assessed symptom severity after treatment for head and neck cancer. A correlative cross sectional study of the DAHANCA toxicity scoring system and the EORTC quality of life questionnaires. Radiother Oncol. 2006;78(3):298–305.
- Falchook AD, Green R, Knowles ME, et al. Comparison of patient- and practitioner-reported toxic effects associated with chemoradiotherapy for head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2016;142(6):517–523.
- Kluetz PG, Chingos DT, Basch EM, et al. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the national cancer institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book. 2016;35:67–73.
- Chera BS, Eisbruch A, Murphy BA, et al. Recommended patient-reported core set of symptoms to measure in head and neck cancer treatment trials. J Natl Cancer Inst. 2014;106(7):pii: dju127.
- Boyes H, Barraclough J, Ratansi R, et al. Structured review of the patient-reported outcome instruments used in clinical trials in head and neck surgery. Br J Oral Maxillofac Surg. 2018;56(3):161–167.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. JCO. 2016;34(6):557–565.
- Olsen MH, Bøje CR, Kjaer TK, et al. Socioeconomic position and stage at diagnosis of head and neck cancer – a nationwide study from DAHANCA. Acta Oncologica (Stockholm, Sweden). 2015;54(5):759–766.
- Hollander-Mieritz C, Vogelius I, Kristensen C, et al. Management of side effects in head and neck cancer by systematic use of PRO during radiotherapy-The national DAHANCA PRO study-DAHANCA 38-Protocol version 1.3 2019; [cited 2019 Dec 2]. Available from: https://www.dahanca.dk/assets/files/PRO_DAHANCA%2038.pdf.
- Danish Head and Neck Cancer Group. DAHANCA Radiotherapy Guidelines 2018; [cited 2019 Dec 2]. Available from: https://dahanca.dk/assets/files/GUID_DAHANCA_Radiotherapy%20guidelines%202018.pdf.
- Hollander-Mieritz C, Johansen C, Vogelius I, et al. 26th Annual Conference of the International Society for Quality of Life Research. Qual Life Res. 2019;28(1):179–180.
- Hollander-Mieritz C, Johansen J, Johansen C, et al. Comparing the patients’ subjective experiences of acute side effects during radiotherapy for head and neck cancer with four different patient-reported outcomes questionnaires. Acta Oncologica (Stockholm, Sweden). 2019;58:603–609.
- Sandler KA, Mitchell SA, Basch E, et al. Content validity of anatomic site-specific Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) item sets for assessment of acute symptomatic toxicities in radiation oncology. Int J Radiat Oncol Biol Phys. 2018;102(1):44–52.
- Hjollund NH, Larsen LP, Biering K, et al. Use of Patient-Reported Outcome (PRO) measures at group and patient levels: experiences from the generic integrated PRO system, WestChronic. Interact J Med Res. 2014;3(1):e5.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- Tolstrup LK, Pappot H, Zangger G, et al. Danish translation, cultural adaption and initial psychometric evaluation of the patient feedback form. Health Qual Life Outcomes. 2018;16(1):77.
- Snyder CF, Blackford AL, Wolff AC, the Patient Viewpoint Scientific Advisory Board, et al. Feasibility and value of PatientViewpoint: a web system for patient-reported outcomes assessment in clinical practice. Psycho-oncology. 2013;22(4):895–901.
- Center for Open Science. Statistical analysis plan for DAHANCA 38 2019 [cited 2019 Dec 2]. Available from: https://osf.io/kqjyr/.
- EORTC Quality of Life Group. Guidelines for assessing Quality of Life in EORTC clinical trials 2002 [cited 2019 Dec 2]. Available from: https://www.eortc.org/app/uploads/sites/2/2018/02/clinical_trials__guidelines_qol.pdf.