Abstract
Background: Post-transplant lymphoproliferative disorder (PTLD) is a rare but life-threatening complication of transplantation. For refractory and relapsed PTLD new therapies are needed, such as the antibody-drug conjugate brentuximab vedotin that targets CD30. There is limited knowledge of CD30 expression in various subtypes of PTLD and its correlation to clinicopathological features. Therefore, we studied the expression of CD30 in PTLD following solid organ transplantation and correlated CD30 expression to PTLD subtype, Epstein-Barr virus (EBV)-status, intratumoral regulatory T-cells (Tregs), clinical features, and outcome.
Methods: We included 50 cases of PTLD from a nation-wide study of PTLDs following solid organ transplantation in Sweden. The tumor biopsies were reevaluated, and clinical data were collected. CD30 expression on tumor cells was analyzed by immunohistochemistry with the clone Ber-H2. Thirty-one cases were stained with clone 236 A/E7 for detection of forkhead box protein 3 (FoxP3, a Treg biomarker).
Results: The case series consisted of 6% polymorphic, 88% monomorphic, and 6% Hodgkin lymphoma-like PTLDs and 53% of the cases were EBV+. Overall, 70% (35/50) of the PTLDs were CD30+ (≥1% CD30+ tumor cells) and 30% (15/50) were CD30-. All polymorphic PTLDs (n = 3) and Hodgkin lymphomas (n = 3), 88% (14/16) of non-germinal center type of diffuse large B-cell lymphoma (DLBCL), and 75% (9/12) of T-cell PTLDs were CD30+ whereas all germinal center-type of DLBCL (n = 5) and Burkitt type PTLD (n = 2) were CD30-. CD30+ PTLD tended to be EBV+ more frequently (p = .07) and occurred earlier posttransplant (2.1 vs. 8.2 years, p = .01) than CD30- PTLD. Type of transplant and localization of the tumor did not differ between the groups except that CNS engagement was more common in CD30- PTLD (p = .02). CD30-status was not associated with presence of intratumoral Tregs or overall survival.
Conclusion: Expression of CD30 varied with PTLD subtype. There was no association between CD30 and survival, regardless of subtype.
Introduction
Post-transplant lymphoproliferative disorders (PTLD) are a heterogeneous group of lymphoid proliferations which may occur after transplantation. They are divided in nondestructive (formerly called early lesions), polymorphic, monomorphic B- and T-cell, and Hodgkin lymphoma-like PTLD [Citation1]. Some cases of PTLD may respond to reduction of immunosuppression alone. Survival has improved substantially since the introduction of rituximab, alone or in combination with chemotherapy [Citation2–4]. Epstein-Barr virus (EBV) can be detected in the tumor cells in about 60% of PTLDs after solid organ transplantation [Citation1,Citation5]. EBV-positive (EBV+) PTLDs may be treated successfully by adoptive immunotherapy with EBV-specific cytotoxic T-cells [Citation6]. However, new treatment options are needed for patients with refractory or relapsed PTLD or when immunotherapy is not feasible.
The CD30-directed antibody-drug conjugate brentuximab vedotin is such a potential new therapy [Citation4]. It is approved for the treatment of relapsed or refractory Hodgkin lymphoma and anaplastic large cell lymphoma (ALCL). The published experience of brentuximab vedotin therapy to patients with PTLD is limited to just over ten patients. According to two phase I/II trials and a couple of case reports the response to brentuximab vedotin is favorable for both monomorphic B- and T-cell PTLD [Citation7–13]. In an ongoing trial, brentuximab vedotin is combined with rituximab as induction and maintenance therapy for lymphomas in immunocompromised patients, including PTLD [Citation4,Citation8]. Preliminary results are promising with a complete response in five of six patients with PTLD [Citation8].
CD30 is a member of the tumor necrosis factor receptor superfamily. Its normal expression is restricted to small subpopulations of activated T- and B- cells. CD30 is further expressed on several lymphomas where CD30 is thought to contribute to lymphomagenesis through promotion of cell proliferation and anti-apoptotic mechanisms [Citation14]. The highest CD30 expression is found in classical Hodgkin lymphoma and ALCL, but CD30 is expressed at lower levels in a subset of other T- and B-cell lymphomas, notably EBV+ diffuse large B-cell lymphoma (DLBCL) and PTLD [Citation14,Citation15]. Approximately 80–90% of PTLDs were reported to express CD30 in previous case series [Citation16–18]. There is not a clear correlation between antitumor effect of brentuximab vedotin and expression of CD30 in lymphoma tissue but a minimum expression threshold appears to be required for antitumor activity [Citation7,Citation19]. Besides being a potential predictive biomarker for response to brentuximab vedotin, expression of CD30 has been reported to be a favorable prognostic marker for both de novo DLBCL and PTLD [Citation15,Citation16]. Furthermore, an association between higher frequency of intratumoral regulatory T-cells (Tregs) and CD30 expression in PTLD was recently reported [Citation18].
There is limited knowledge of CD30 expression in various subtypes of PTLD and its correlation to clinicopathological features. Therefore, we studied the expression of CD30 in 50 cases of PTLD retrieved from a larger population-based case series following solid organ transplantation. We correlated the CD30 expression to PTLD subtype, EBV-status, presence of intratumoral Tregs, clinical features, and outcome.
Material and methods
Study population
From a previously reported population-based case series of 135 lymphomas following solid organ transplantation in Sweden between 1980 and 2006 [Citation5], all cases of PTLD with sufficient representative material for further analysis or with available glass slides of CD30 immunohistochemistry (IHC) from routine diagnostics were included (n = 50). Clinical data were obtained by one physician (AK) from medical records at all transplantation centers in Sweden and from all the hospitals throughout the country where the patients had been followed posttransplant. All study subjects were followed from the date of transplantation until death or end of follow-up (25 October 2012), which-ever occurred first. The study was approved by the Regional Ethical Review Board in Uppsala, Sweden.
Reevaluation of PTLDs
The tumor biopsies have been reevaluated according to the 2008 revision of the WHO classification of lymphoid neoplasms by an experienced hematopathologist (CS) and the diagnoses are consistent with the latest revision of 2016 [Citation1,Citation20]. Cell-of-origin classification of DLBCL into germinal center (GC) or non-GC subtype was based on IHC and the Hans algorithm [Citation21]. EBV-status of PTLD was analyzed by EBV-encoded RNA (EBER) in situ hybridization (ISH). Expression of forkhead box protein 3 (FoxP3), a biomarker of Tregs, was analyzed by IHC with clone 236 A/E7 (dilution 1/100, eBioscience). FoxP3+ cells were counted manually, and the area of the biopsy was calculated with the preinstalled software of the microscope (Leica Microsystems Inc.). A positive case was defined as ≥29 FoxP3+ cells/mm2 as previously reported [Citation22].
Immunohistochemistry for CD30
The definition of CD30 positivity was ≥1% expression on tumor cells which is the same as used in several clinical trials of brentuximab vedotin in B-cell lymphomas [Citation7]. In cases of polymorphic PTLD, expression on large atypical cells was counted. CD30 was determined by two independent hematopathologists (RMA and PH) by visual assessment of IHC stainings, which were either already available from routine diagnostics (n = 11) or performed as part of this study (n = 39). In cases with divergent results (n = 2) the slides were reevaluated to reach a consensus.
The eleven preexisting immunostainings were performed at Swedish University hospitals between 1995 and 2009. The IHC done as part of the study was performed on 4-μm thick formalin-fixed paraffin-embedded (FFPE) tissue sections with the use of clone Ber-H2 (dilution 1/50, Dako) according to standard protocols mainly with the automated staining system IntelliPATH (Biocare Medical) at the Clinical Research and Development Unit, Uppsala University Hospital or at the Swedish Science for Life Laboratory facilities, Uppsala University, Uppsala, Sweden.
Statistics
Comparisons between groups were performed with the Chi-square test or the Fisher’s exact test for categorical and the Mann-Whitney U-test for continuous variables. The following variables were assessed in statistical analysis: CD30-status, PTLD subtype, EBER- and latent membrane protein-1 (LMP-1)-status of PTLD, age at PTLD diagnosis, sex, type of transplantation (kidney ± pancreas, heart, liver, lung), immunosuppressive regimen before PTLD (ever/never), time from last transplantation to PTLD diagnosis, nodal only vs. extranodal localization of PTLD, involved organs by PTLD, presentation stage according to Ann Arbor (I-III vs. IV), performance status according to Eastern Cooperative Oncology Group (ECOG, 0–1 vs. 2–4), B symptoms at PTLD diagnosis, serum lactate dehydrogenase (LDH) before start of PTLD treatment, age-adjusted International Prognostic Index (IPI), treatment regimens, response to treatment, calendar year of PTLD, and frequency of FoxP3+ cells intratumorally. Overall survival was calculated from the date of PTLD diagnosis to death from any cause by Kaplan-Meier time-to-event analysis. Differences in survival were evaluated using log-rank test and Cox proportional hazards regression. p values < .05 was considered statistically significant. Statistica Software (version 13, TIBCO Software Inc.) was used in all analyses.
Results
This case series of PTLD after solid organ transplantation consisted of 6% polymorphic, 88% monomorphic, and 6% Hodgkin lymphoma-like PTLD. EBV was detected in the tumor microenvironment in 53% of the cases overall, in 100% (3/3) of polymorphic, in 62% (18/29) of B-cell monomorphic, in 8% (1/12) of T-cell monomorphic, and 100% (3/3) of Hodgkin lymphoma-like PTLD.
CD30 and PTLD subtype
Overall, 70% of the PTLDs were CD30+ (n = 35) and 30% were CD30- (n = 15). All polymorphic PTLDs (n = 3) and Hodgkin lymphomas (n = 3), 88% (14/16) of non-GC type of DLBCL, and 75% (9/12) of T-cell PTLDs were CD30+ whereas all GC-type of DLBCL (n = 5) and Burkitt lymphomas (n = 2) were CD30- (). The non-GC type of DLBCL was significantly more often CD30+ than the GC-type (p = .001). CD30+ PTLDs tended to be EBV+ more frequently compared with CD30- cases both when analyzed by EBER ISH (62% vs. 33%, p = .07) and by LMP-1 IHC (69% vs. 38%, p = .20).
Table 1. Clinicopathological characteristics of PTLD cases by CD30 status.
Among the CD30+ PTLDs, the frequency of positive tumor cells varied from one per cent to practically all (). Hodgkin lymphoma and ALCL were the PTLD subtypes that had the highest expression of CD30.
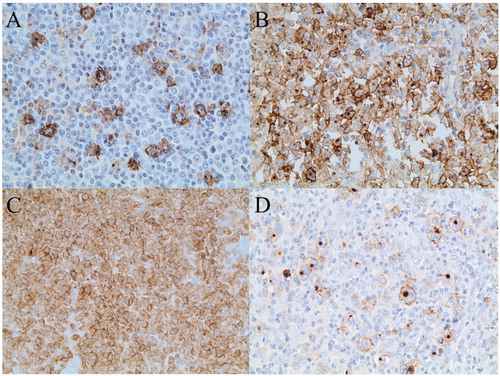
Figure 1. Immunohistochemical staining of CD30 in PTLD after solid organ transplantation. (A) Polymorphic PTLD with approximately 10% CD30+ cells. (B) Diffuse large B-cell lymphoma, non-germinal center type, with approximately 80% CD30+ tumor cells. (C) Anaplastic large cell lymphoma with 100% CD30+ tumor cells D) Hodgkin lymphoma-like PTLD with 100% CD30+ Hodgkin and Reed/Sternberg (HRS) cells.
CD30 and clinical features
There was no difference in age, gender, type of transplant, or immunosuppressive regimen before PTLD diagnosis between patients with CD30+ and CD30- PTLDs (). The CD30+ PTLDs occurred earlier after transplantation than the CD30- cases (2.1 vs. 8.2 years posttransplant, p = .01). A majority of both CD30+ and CD30- PTLD had extranodal localization (74% vs. 93%), most commonly occurring in the gastrointestinal tract. The frequency of tumor engagement of the allograft was similar in CD30+ and CD30- cases (14% vs. 13%). Engagement of the central nervous system was more common in CD30- PTLD (20% vs. 0%, p = .02) but numbers were small.
Patients with CD30- PTLD tended to be at a more advanced stage at diagnosis (69% vs. 35% Ann Arbor stage IV, p = .05), to more frequently have elevated serum LDH (92% vs. 65%, p = .12), and a higher age-adjusted IPI (73% vs. 40% 2–3 points, p = .09) compared with patients with CD30+ PTLD. There was no difference in performance status according to ECOG or presence of B symptoms at PTLD diagnosis between patients with CD30+ and CD30- PTLD.
CD30 and outcome
Treatment modalities were heterogeneous but did not differ between the two groups (). There was no difference in response to treatment (52% vs. 69% complete response, p = .33) or in overall survival between patients with CD30+ and CD30- PTLD (, log-rank test, p = .42). Rituximab did not improve survival in monomorphic B-cell PTLD in this case series, but the total number of rituximab treated patients was low.
Figure 2. Overall survival in PTLD after solid organ transplantation by CD30-status overall (A), in the subgroup of DLBCL (B), and the subgroup of T-cell PTLD (C).
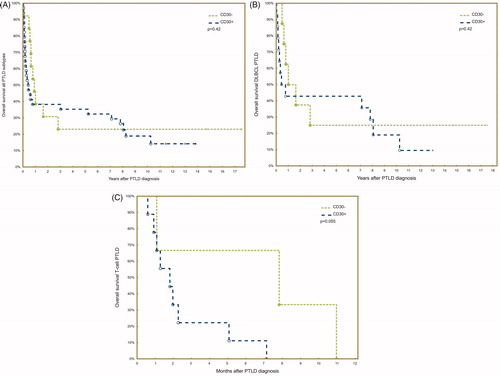
Since the outcome differed considerably between monomorphic B- and T-cell PTLD, we also performed survival analysis separately for T-cell PTLD and DLBCL, which was the largest subgroup of monomorphic B-cell PTLD. CD30-status was not associated with overall survival within the subgroup of DLBCL (, log-rank test, p = .42). However, CD30- T-cell PTLDs tended to have a superior overall survival compared with CD30+ T-cell PTLDs (, log-rank test, p = .06) but the result must be interpreted with caution since there were only three CD30- T-cell PTLDs in the analysis.
Furthermore, using Cox proportional hazards regression, CD30-status was not associated with overall survival in the whole case series of PTLD, neither in univariate nor in multivariate analysis (adjusting for age, sex, and stage) (). Nor in the subgroups of DLBCL and T-cell PTLD respectively did CD30-status have an impact on overall survival in multivariate analysis.
Table 2. Uni- and multivariate analysis of CD30 and overall survival with the use of Cox proportional hazards regression, adjusting for age, sex, and stage according to Ann Arbor.
CD30 and FoxP3
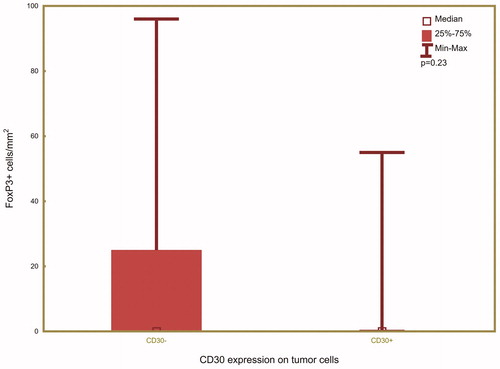
A subgroup of 31 PTLDs was also stained for FoxP3, which is a biomarker for Tregs. Among the CD30+ PTLDs, four (20%) were FoxP3+ and 16 (80%) were FoxP3-. Among the CD30- PTLDs, five (45%) were FoxP3+ and six (55%) were FoxP3-. There was no significant association between the frequency of intratumoral FoxP3+ cells/mm2 and CD30 expression in PTLD (, p = .23).
Discussion
In this nation-wide case series of 50 PTLDs after solid organ transplantation, 70% of the PTLDs expressed CD30 in the tumor cells and were thus potentially treatable with brentuximab vedotin. Previous case series have reported that 79–87% of the PTLDs were CD30+ [Citation16–18]. In line with previous studies, we found that all Hodgkin lymphomas and polymorphic PTLDs and a vast majority of non-GC-type of DLBCLs and T-cell PTLDs were CD30+, whereas GC-type of DLBCLs and Burkitt lymphomas were all CD30- [Citation16,Citation17,Citation23–25]. Furthermore, CD30+ PTLDs have been associated with EBV-positivity by EBER ISH [Citation16–18]. We saw a trend that CD30+ PTLDs were more frequently EBV+ either when analyzed by EBER ISH or by LMP-1 IHC but numbers were too small to prove an association. We report for the first time that CD30+ PTLDs occur significantly earlier post-transplant than CD30- cases, which was seen previously as a trend in a study by Hartley et al. [Citation18].
The somewhat lower frequency of CD30+ PTLDs in our compare to previous case series may in part be explained by different composition of PTLD subtypes in the studies [Citation16–18]. As a consequence of the long follow-up time in our study (almost three decades), a small proportion of the PTLDs were early-onset polymorphic PTLD (6%) and a large proportion were EBV-negative monomorphic PTLD (47%). Clearly, our case series is not comparable with the one by Schober et al. with 98% EBV+ PTLDs in 63 children [Citation17]. Also in the case series of 62 PTLDs by Vase et al. there was a higher proportion of EBV+ PTLD (85% vs. 53%) and nondestructive or polymorphic lesions (30% vs. 6%) than we had [Citation16]. In the study by Hartley et al. there were 25 patients who had undergone solid organ transplantation and eight patients after bone marrow transplantation (all of whom had CD30+ PTLDs) which impedes a direct comparison [Citation18]. Furthermore, there was a higher proportion of polymorphic PTLD (21%) and EBV+ PTLD (76%) than in our study.
Vase et al. reported that CD30-positivity was an independent favorable prognostic factor in PTLD regardless of EBV-status [Citation16]. CD30 expression has been associated with a favorable prognosis also in de novo DLBCL in immunocompetent individuals, but this held true only for EBV-negative cases since CD30+ EBV+ DLBCLs had a particularly poor outcome [Citation15]. Schober et al. found a trend toward a favorable outcome in CD30+ PTLD and Hartley et al. showed no impact of CD30-status in PTLD on overall survival [Citation17,Citation18]. In our case series there was no difference in overall survival between CD30+ and CD30- PTLDs overall. We and others have previously shown that T-cell PTLD have a particularly poor prognosis [Citation5,Citation26,Citation27]. In this case series, none of the 12 patients with T-cell PTLD survived a year. Therefore, we analyzed survival of DLBCLs and T-cell PTLD separately. However, CD30-status did not have an impact on survival in either of these subgroups in multivariate analysis. Apart from differences in composition of PTLD subtypes between the CD30+ and CD30- group, the heterogeneous therapy modalities may have affected the survival analysis.
Recently, Hartley et al. reported that intratumoral Tregs frequencies were higher in CD30+ than in CD30- PTLDs, which fits well with the mechanism that CD30 normally induces proliferation of Tregs [Citation18]. We could not confirm this finding; on the contrary the frequency of intratumoral Tregs tended to be higher in CD30- PTLD in our case series. However, this result must be interpreted with caution since there were only nine FoxP3+ cases. In part this discrepancy may be explained by the fact that one third of the CD30+ PTLDs in our subgroup stained for FoxP3 was T-cell lymphomas whereas in the case series by Hartley et al. there were no T-cell PTLDs [Citation18]. In a previous study including partly the same individuals as in this present case series, we explored factors influencing FoxP3 expression in PTLD by a multivariate analysis (general discriminant analysis) and found that T-cell PTLD was associated with FoxP3-negativity (p = .006) [Citation22]. We also showed that the frequency of Tregs in DLBCL-type PTLD was substantially lower than the frequency reported in DLBCL in immunocompetent individuals, probably due to the immunosuppressive drugs taken to prevent or treat allograft rejection [Citation22,Citation28]. This finding was confirmed by Hartley et al. and by another study of Tregs in PTLD [Citation18,Citation29].
We chose the threshold of ≥1% CD30+ tumor cells as definition of a positive case, whereas the previous studies of CD30 expression in PTLD used >20% [Citation16] or any CD30 expression in tumor cells [Citation17,Citation18]. Universally accepted diagnostic cutoffs for CD30-positivity only exists for ALCL [Citation14]. In a phase 2 trial of brentuximab vedotin for relapsed/refractory DLBCL (n = 49) and other B-cell lymphomas (n = 19) including three PTLDs, CD30 positivity was defined as ≥1% expression on neoplastic cells [Citation7]. In this trial there was no correlation between level of CD30 expression and response, however, all responding patients had CD30+ tumors assessed by computer-assisted digital image analysis. It has also been reported that patients with CD30- DLBCL achieved complete remission on brentuximab vedotin [Citation19]. However, a majority of (11 of 16) these tumor samples deemed CD30- by visual inspection had quantifiable low grade CD30 expression by computer-assisted digital image analysis, which is a more sensitive method.
In summary, in this nation-wide case series of PTLD after solid organ transplantation with a large proportion of late-onset, monomorphic, EBV-negative PTLD we found that the frequency of CD30 expression was somewhat lower compared to previous case series with a majority of EBV+ PTLD. A clear majority of non-GC DLBCL and T-cell PTLDs, and all polymorphic and Hodgkin lymphoma-like PTLDs were CD30+ whereas GC-type of DLBCL and Burkitt lymphomas were all CD30-. CD30+ PTLD was associated with early onset after transplantation. The compound brentuximab vedotin may find a therapeutic role in refractory or relapsed PTLD in certain subtypes of PTLD in the future.
Author contributions
AK, CS, DM, and GE designed the study, AK collected the clinical data, CS reevaluated the PTLD diagnoses, RMA and PH analyzed the CD30 stainings, AK analyzed the results and wrote the paper, which all authors critically reviewed and finally approved.
Disclosure statement
DM has received honoraria from Roche, Merck, Bristol-Myers Squibb, and Takeda. The other authors report no conflicts of interest.
Additional information
Funding
References
- Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378(6):549–562.
- Trappe R, Oertel S, Leblond V, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196–206.
- Reshef R, Vardhanabhuti S, Luskin MR, et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder. Am J Transplant. 2011;11(2):336–347.
- DeStefano CB, Desai SH, Shenoy AG, et al. Management of post-transplant lymphoproliferative disorders. Br J Haematol. 2018;182(3):330–343.
- Kinch A, Baecklund E, Backlin C, et al. A population-based study of 135 lymphomas after solid organ transplantation: the role of Epstein-Barr virus, hepatitis C and diffuse large B-cell lymphoma subtype in clinical presentation and survival. Acta Oncol. 2014;53(5):669–679.
- Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–1131.
- Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394–1402.
- Gandhi M, Smith SM, Nabhan, C, et al. Brentuximab vedotin (BV) plus rituximab (R) as frontline therapy for patients (pts) with epstein barr virus (EBV)+ and/or CD30+ lymphoma: phase I results of an ongoing phase I–II study. Blood. 2014;124(21):3096–3096.
- Hill BT, Tubbs RR, Smith MR. Complete remission of CD30-positive diffuse large B-cell lymphoma in a patient with post-transplant lymphoproliferative disorder and end-stage renal disease treated with single-agent brentuximab vedotin. Leuk Lymphoma. 2015;56(5):1552–1553.
- Choi M, Fink S, Prasad V, et al. T Cell PTLD successfully treated with single-agent brentuximab vedotin first-line therapy. Transplantation. 2016;100(3):e8–e10.
- Schaefer B, Steurer M, Glodny B, et al. First experience with brentuximab vedotin in posttransplant lymphoproliferative disorder after liver transplantation: complete remission followed by lethal sepsis. Liver Transpl. 2014;20(9):1145–1148.
- Berger GK, McBride A, Lawson S, et al. Brentuximab vedotin for treatment of non-Hodgkin lymphomas: a systematic review. Crit Rev Oncol Hematol. 2017;109:42–50.
- Mika T, Strate K, Ladigan S, et al. Refractory Epstein-Barr Virus (EBV)-related post-transplant lymphoproliferative disease: cure by combined brentuximab vedotin and allogeneic EBV-specific T-lymphocytes. Front Med (Lausanne)). 2019;6:295.
- van der Weyden CA, Pileri SA, Feldman AL, et al. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J. 2017;7(9):e603–e603.
- Hu S, Xu-Monette ZY, Balasubramanyam A, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2013;121(14):2715–2724.
- Vase MO, Maksten EF, Bendix K, et al. Occurrence and prognostic relevance of CD30 expression in post-transplant lymphoproliferative disorders. Leuk Lymphoma. 2015;56(6):1677–1685.
- Schober T, Framke T, Großhennig A, et al. CD30 in pediatric post-transplant lymphoproliferative disease after solid organ transplant: characterization of a new therapeutic target. Leuk Lymphoma. 2015;56(3):832–833.
- Hartley C, Vaughan JW, Jarzembowski J, et al. CD30 expression in monomorphic posttransplant lymphoproliferative disorder, diffuse large B-cell lymphoma correlates with greater regulatory T-cell infiltration. Am J Clin Pathol. 2017;148(6):485–493.
- Bartlett NL, Smith MR, Siddiqi T, et al. Brentuximab vedotin activity in diffuse large B-cell lymphoma with CD30 undetectable by visual assessment of conventional immunohistochemistry. Leuk Lymphoma. 2017;58(7):1607–1616.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390.
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282.
- Berglund D, Kinch A, Edman E, et al. Expression of intratumoral forkhead box protein 3 in posttransplant lymphoproliferative disorders: clinical features and survival outcomes. Transplantation. 2015;99(5):1036–1042.
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of tumours of haematopoietic and lymphoid tissues. Lyon: World Health Organization; 2017.
- Kampers J, Orjuela-Grimm M, Schober T, et al. Classical Hodgkin lymphoma-type PTLD after solid organ transplantation in children: a report on 17 patients treated according to subsequent GPOH-HD treatment schedules. Leuk Lymphoma. 2017;58(3):633–638.
- Malysz J, Erdman P, Klapper J, et al. Clinical implications of CD30 expression in aggressive B-cell lymphomas. Clin Lymphoma Myeloma Leuk. 2016;16(8):429–433.
- Koff JL, Li JX, Zhang X, et al. Impact of the posttransplant lymphoproliferative disorder subtype on survival. Cancer. 2018;124(11):2327–2336.
- Caillard S, Porcher R, Provot F, et al. Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol. 2013;31(10):1302–1309.
- Tzankov A, Meier C, Hirschmann P, et al. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma [Multicenter Study]. Haematologica. 2008;93(2):193–200.
- Richendollar BG, Tsao RE, Elson P, et al. Predictors of outcome in post-transplant lymphoproliferative disorder: an evaluation of tumor infiltrating lymphocytes in the context of clinical factors. Leuk Lymphoma. 2009;50(12):2005–2012.