Abstract
Introduction: Tumor-associated trypsin inhibitor (TATI) limits serine proteases, promotes carcinogenesis in several cancers and functions as an acute-phase reactant. Tumor-associated trypsin-2 (TAT-2), a proteolytic target enzyme for TATI, can enhance invasion by promoting extracellular matrix degradation. Here, we aimed to study serum TATI and TAT-2 levels, including the TAT-2/TATI ratio, as prognostic and diagnostic biomarkers in gastric cancer. We compared the results with the plasma level of C-reactive protein (CRP).
Material and Methods: We selected 240 individuals operated on for gastric adenocarcinoma at the Helsinki University Hospital, Finland, between 2000 and 2009. We determined the preoperative serum TAT-2, TATI and plasma CRP levels using time-resolved immunofluorometric assays using monoclonal antibodies.
Results: The medium serum TAT-2 level was higher among gastric cancer patients [8.68 ng/ml; interquartile range (IQR) 5.93–13.2] than among benign controls (median 5.41 ng/ml; IQR 4.12–11.8; p = .005). Five-year survival among patients with a high serum TAT-2 was 22.9% [95% confidence interval (CI) 11.7–34.1], compared to 52.2% (95% CI 44.6–59.8; p < .001) among those with a low level. The five-year survival among patients with a high serum TATI was 30.6% (95% CI 20.4–40.8), compared to 52.9% (95% CI 44.7–61.1; p < .001) among those with a low level. The serum TATI level remained significant in the multivariable survival analysis (hazard ratio 2.01; 95% CI 1.32–3.07). An elevated plasma CRP level associated with a high serum TATI level (p = .037).
Conclusions: This study shows for the first time that a high serum TAT-2 may function as a prognostic biomarker in gastric cancer and that TAT-2 levels may be elevated compared to controls. Additionally, we show that the prognosis is worse among gastric cancer patients with a high serum TATI. These biomarkers serve as prognostic factors particularly among patients with a metastatic or a locally advanced disease.
Introduction
Gastric adenocarcinoma represents one of the most lethal cancers worldwide [Citation1]. In the early stages of the disease, symptoms are scarce; when severe symptoms appear, however, the disease has often already spread. None of the tumor markers currently clinically available have demonstrated a sufficient sensitivity to be useful for detecting gastric cancer and carry a limited prognostic value [Citation2].
Tumor-associated trypsin inhibitor (TATI), also known as the pancreatic secretory trypsin inhibitor (PSTI), is a serine protease inhibitor first found in the urine of a patient with gynecological cancer [Citation3,Citation4], called serine peptidase inhibitor Kazal-type 1 (SPINK1). In addition to inhibiting serine proteases, it appears to promote carcinogenesis by activating the epidermal growth factor receptors (EGFRs) [Citation5,Citation6]. In addition to gastric cancer, TATI expresses in several other cancers, including colorectal, pancreatic, ovarian, bladder, renal, prostate, lung, breast and liver cancers, and appears useful for both diagnostic and prognostic purposes [Citation6]. The prognostic role of TATI in gastric cancer remains controversial since, on the one hand, a high TATI expression in gastric carcinoma tissues served as a marker of a favorable outcome and associated with a low cancer stage, superficial tumors and the absence of metastasis. On the other hand, higher serum TATI levels were found more often among gastric cancer patients than among controls and associated with advanced disease [Citation7,Citation8]. Additionally, TATI acts as an acute-phase reactant and the regulation of its synthesis resembles that of the C-reactive protein (CRP) and other acute-phase reactants [Citation9,Citation10]. Thus, in addition to malignant diseases, an elevated serum TATI level may result from an inflammation caused by an infection or tissue damage [Citation11,Citation12].
Tumor-associated trypsin-2 (TAT-2) represents one of the proteolytic target enzymes for TATI [Citation13]. A high TAT-2 expression enhances a tumor’s invasive capacity through increased extracellular matrix degradation via the activation of pro-urokinase and matrix metalloproteinases [Citation14,Citation15]. Moreover, a surplus of TAT-2 compared to TATI, leading to a high TAT-2/TATI ratio, may result in increased TAT-2 activity [Citation6]. Furthermore, TAT-2 expresses in several cancers, including gastric cancer, yet the background levels of pancreatic secretory trypsin limit its clinical use in diagnosing pancreatitis [Citation16–19]. In a previous study of serum trypsinogen in gastric cancer patients, TAT-2 levels were significantly higher among those with a linitis plastica–type gastric cancer compared to those with a non-linitis plastica-type gastric cancer [Citation20]. Likewise, trypsin production by gastric cancer cells associates with an increased in vitro invasiveness of the tumor cells [Citation17,Citation21]. Serum TAT-2 has not been previously studied in gastric cancer as a prognostic biomarker.
CRP has been studied in several cancers in attempts to clarify the mechanism behind the systemic inflammatory reaction sometimes seen in cancer [Citation22]. In colon cancer, after excluding patients undergoing emergency surgery and those with auto-immune disease or infection, an elevated preoperative CRP predicts a poor outcome [Citation23]. A high preoperative CRP was also shown to indicate a poor outcome following surgical resection of pancreatic ductal adenocarcinoma or colorectal liver metastases [Citation24,Citation25]. Among gastric cancer patients, an elevated preoperative CRP served as a marker of a poor outcome and associated with metastatic disease [Citation26,Citation27]. Similarly, a recent meta-analysis consisting of 2597 gastric cancer patients concluded that an elevated preoperative CRP predicts a poor outcome [Citation28].
Here, we aimed to study serum TATI and TAT-2 levels, including the TAT-2/TATI ratio, as prognostic and diagnostic biomarkers in gastric cancer. We also compared these with gastric cancer patients’ plasma CRP levels.
Material and methods
Patients and ethics
Our cohort consisted of 240 patients operated on for gastric adenocarcinoma in the Department of Surgery, Helsinki University Hospital, Finland, between 2000 and 2009, as previously described [Citation29]. Patients undergoing surgery were consecutively included in this study, excluding individuals with a history of malignant disease or synchronous cancer. Each gastric tumor was histologically verified as gastric adenocarcinoma by an experienced pathologist from the Department of Pathology. We used the seventh version of the Tumor-Node-Metastasis (TNM) classification for the cancer staging [Citation30]. We compared gastric cancer patients to 48 control patients undergoing surgery or gastroscopy to treat and diagnose a variety of nonmalignant conditions between 2000 and 2012 (Supplementary Table S1).
We updated survival data in August 2019. Data were received from patient records, the Population Register Center of Finland and Statistics Finland.
The Surgical Ethics Committee of Helsinki University Hospital (Dnro HUS 226/E6/06, extension TMK02 §66 17.4.2013) approved the study protocol. We were authorized to study the archived tissue samples retrospectively without requiring further individual consent or permission as issued by the National Supervisory Authority of Welfare and Health (Valvira Dnro 10041/06.01.03.01/2012).
Serum and plasma samples
Blood samples were drawn 1 to 30 days (median, 1 day) before surgery. Serum and plasma were separated, aliquoted and stored at −80 °C until analysis, according to the routine procedures of the hospital laboratory.
To determine the gastric cancer patients’ plasma CRP levels, we applied a high-sensitivity method [time-resolved immunofluorometric assay (TR-IFMA) in microtitration plates using a monoclonal CRP antibody (anti-hCRP, code 6405, Medix Biochemica, Espoo, Finland)], as detailed elsewhere [Citation24]. The gastric cancer patients’ serum TATI and TAT-2 levels were also determined using TR-IFMA, which we described previously [Citation31,Citation32].
Statistical analyses
We explored the strength of associations between biomarker levels and clinicopathological variables among gastric cancer patients using the Mann–Whitney U-test and the Kruskal–Wallis test. Correlations were evaluated using the Spearman’s rank correlation. We dichotomized the variables for the survival analyses. The cutoff value for CRP was 10 mg/l. In the survival analyses, cutoff values for TAT-2, TATI, and the TAT-2/TATI ratio were determined using the maximum value for Youden’s index to find the best prognostic performance among our cohort of gastric cancer patients [Citation33]. Furthermore, validation analyses were conducted using median cutoff values for TAT-2 and TATI (Supplementary Table S4). We determined the significant difference in biomarker levels between gastric cancer patients and controls using the Mann–Whitney U-test. Additionally, in studying the diagnostic value of TAT-2, the receiver operating characteristic (ROC) curve analysis was applied to study the area under the curve and to determine a distinct cutoff value for TAT-2 to calculate the sensitivity, specificity, predictive values, and accuracy. The cutoff value was determined by the maximum value of Youden’s index to optimally differentiate gastric cancer patients from controls, with equal values for sensitivity and specificity. The Kaplan–Meier method was applied to create survival curves, and the statistical significance was calculated using the log-rank test. We used the Cox proportional hazard model to calculate the hazard ratios (HRs) for the uni- and multivariable survival analyses. Covariates entered into the multivariable survival analysis consisted of age, stage, the Laurén classification and the serum TAT-2 and TATI levels. Cancer stage was processed as a categorical covariate, in which we compare patients with higher cancer stages to those with stage I cancer. Furthermore, we studied gastric cancer patients’ survival in several subgroups using the Cox proportional hazard model and visually depicted it using the Kaplan–Meier estimators. The patient subgroups analyzed were stratified by age, gender, TNM-classification, stage, the Laurén classification, and the CRP, TAT-2, and TATI levels. The primary outcome in all of the survival analyses was death due to gastric cancer. We calculated the disease-specific survival from the date of surgery to the end of follow-up or death due to gastric cancer. Individuals leaving the study before the end of follow-up and individuals dying of causes other than gastric cancer were censored. We considered a two-tailed p-value of less than .05 as statistically significant across all analyses. We used IBM’s SPSS Statistics for Mac version 24.0 (IBM Corporation, Armonk, NY, USA) for all analyses.
Results
Diagnostic value of TAT-2, TATI, TAT-2/TATI ratio and CRP
Serum TAT-2 levels were higher among gastric cancer patients [median 8.68 ng/ml; interquartile range (IQR) 5.93–13.2] than controls (median 5.41 ng/ml; IQR 4.12–11.8; p = .005; Supplementary Table S2). The area under the ROC curve was 0.63 (95% CI 0.53–0.72; p = .005). The maximum value of Youden’s index was used to determine the optimal cutoff value (5.45 ng/ml) for TAT-2 to calculate the sensitivity, specificity, predictive values and accuracy among gastric cancer patients and controls. The sensitivity of TAT-2 to distinguish gastric cancer patients from controls reached 78.3% with a specificity of 52.1%. The positive predictive value reached 89.1%, with a negative predictive value of 32.5% and an accuracy of 74.0%. The prevalence of gastric cancer in this test population reached 83.3%. We found no significant differences in the TATI or CRP levels or in the TAT-2/TATI ratio between gastric cancer patients and controls.
Associations between TAT-2, TATI, TAT-2/TATI ratio, CRP and clinicopathological variables
We found statistically significant associations between a high serum TAT-2 level and pT3–4 tumors (p = .046) and between high serum TAT-2 and TATI levels (p < .001; ). We also identified statistically significant associations between a high serum TATI and an older age (p = .001), between a high serum TATI and pT3–4 tumors (p = .030) and between a high serum TATI and an elevated CRP (p = .037). Furthermore, we discovered statistically significant associations between a high serum TAT-2/TATI ratio and diffuse-type tumors (p = .004) and between a high serum TAT-2/TATI ratio and a younger age (p < .001; Supplementary Table S3). We also identified a positive correlation between the TAT-2 and TATI levels (rs 0.438; p < .001).
Table 1. Association of serum TAT-2 and TATI with the clinicopathologic variables and CRP among 240 gastric cancer patients.
Plasma CRP was elevated among older patients (p = .024), patients with pT3–4 tumors (p = .047) and among patients with intestinal-type tumors (p = .006; Supplementary Table S3). We found no other statistically significant associations between the studied biomarkers and the clinicopathological variables.
Survival analyses
Optimal cutoff values for the survival analyses for TAT-2, TATI and the TAT-2/TATI ratio were determined using the maximum value for Youden’s index (for TAT-2, 12.91 ng/ml; for TATI, 18.76 ng/ml; and for the TAT-2/TATI ratio, 0.58). Five-year disease-specific survival among patients with a high serum TAT-2 was 22.9% [95% confidence interval (CI) 11.7–34.1], and 52.2% among patients with a low TAT-2 (95% CI 44.6–59.8; p < .001; ; ). The five-year survival among patients with a high serum TATI was 30.6% (95% CI 20.4–40.8), and 52.9% among patients with a low TATI (95% CI 44.7–61.1; p < .001; ). The serum TAT-2/TATI ratio or plasma CRP did not function as significant prognostic factors in the whole cohort.
Figure 1. Gastric cancer patients’ disease-specific survival according to the Kaplan-Meier method. P-values were calculated according to the log-rank test. Low versus high (A) TAT-2 and (B) TATI across the entire cohort. Low versus high (C) TAT-2 and (D) TATI among patients with pT3–4 tumors. (E) Low versus high TAT-2 among patients with a low TATI and (F) low versus high TATI among patients with a low TAT-2.
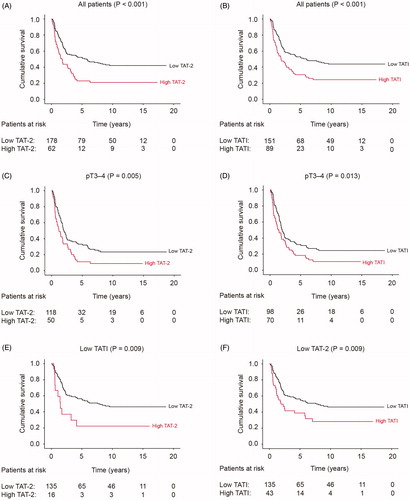
Table 2. Uni- and multivariable survival analyses for 240 gastric cancer patients.
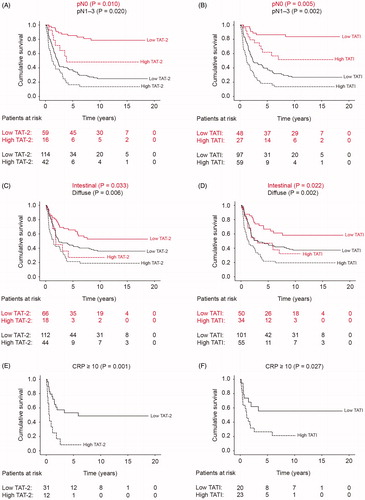
Serum TATI remained an independent prognostic factor in the multivariable survival analysis [hazard ratio (HR) 2.01; 95% CI 1.32–3.07; ]. Other significant prognostic factors in the multivariable survival analysis included age, stage and histological type according to the Laurén classification. No significant interaction terms were identified in the final model.
Survival analyses by subgroups
Among patients with pT3–4 tumors, a high TAT-2 level () and a high TATI level (; ) served as markers of a poor outcome. A high TAT-2 level served as a marker for an adverse outcome among those with a low TATI level (). Similarly, a high TATI level served as a marker for a poor outcome among those with a low TAT-2 level ().
Table 3. Survival analyses by subgroups, high serum TAT-2 and TATI levels compared to low among 240 gastric cancer patients.
Furthermore, a high TAT-2 level served as a marker for a poor outcome among those with lymph-node metastasis and among those without lymph-node metastasis (; ). Correspondingly, a high TATI level served as a marker for a poor outcome among those with lymph-node metastasis and among those without lymph-node metastasis (). A high TAT-2 level emerged as indicative of a poor outcome among those with either intestinal- or diffuse-type tumors (). Likewise, a high TATI level served as a marker for a poor outcome among those with either intestinal- or diffuse-type tumors (). A high TAT-2 level served as a marker for a poor outcome among those with an elevated CRP () and among those without an elevated CRP. Similarly, a high TATI level served as a marker for a poor outcome among those with an elevated CRP () and among those without an elevated CRP. Additionally, TAT-2 and TATI served as prognostic biomarkers in several other subgroups presented in .
Figure 2. Gastric cancer patients’ disease-specific survival stratified by subgroups according to the Kaplan–Meier method. p-Values were calculated according to the log-rank test. Low versus high (A) TAT-2 and (B) TATI among patients with lymph-node metastasis (red lines) and among patients without lymph-node metastasis (black lines). Low versus high (C) TAT-2 and (D) TATI among patients with intestinal- (red lines) and diffuse-type (black lines) tumors. Low versus high (E) TAT-2 and (F) TATI among patients with elevated CRP levels.
A high TAT-2/TATI ratio served as a marker for a poor outcome among older patients (HR 2.26; 95% CI 1.39–3.67).
Discussion
Here, we show that, in our cohort of 240 gastric adenocarcinoma patients, high serum TAT-2 and TATI levels function as markers for a poor outcome. Both parameters are promising prognostic biomarkers, although only TATI remained significant in the multivariable survival analysis, suggesting its prognostic value is superior to that of TAT-2 in this patient cohort. Nevertheless, to our knowledge, this is the first time that serum TAT-2 has been identified as a prognostic biomarker in gastric cancer. Additionally, we show that the expression levels of these biomarkers correlate positively with gastric cancer. Neither the TAT-2/TATI ratio nor CRP functioned as prognostic biomarkers in this study. Furthermore, we evaluated the biomarkers’ diagnostic properties, demonstrating that serum TAT-2 levels were higher among gastric cancer patients than among benign controls.
High serum TAT-2 and TATI levels predicted an unfavorable outcome in several of the subgroups we studied. Interestingly, they did not function as prognostic factors among patients with pT1–2 tumors or with stage I, II or III disease, but among those with pT3–4 tumors or stage IV disease. Because high TAT-2 and TATI levels were also associated with pT3–4 tumors, the mechanisms via which a high level of TAT-2 or TATI affect disease progression appear important in locally advanced or metastatic disease rather than in early-stage gastric cancer. Specifically, TAT-2 enhances the tumor’s invasive abilities via the increased degradation of the extracellular matrix through the activation of pro-urokinase and matrix metalloproteinases. TATI, furthermore, promotes tumor growth via the activation of EGFRs. In a clinical setting, our results agree with previous studies [Citation6,Citation14,Citation15]. However, because TAT-2 and TATI also functioned as prognostic factors among patients without lymph-node or distant metastases, their roles in gastric cancer carcinogenesis do not appear limited to the pathogenesis of advanced disease.
This study further clarifies the role of TATI in gastric cancer, as previously described, as associating, on the one hand, with advanced disease and, on the other hand, associating with a favorable outcome and the absence of metastasis [Citation7,Citation8]. A high tissue expression of TATI was previously found to associate with a favorable prognosis, superficial tumors and the absence of lymph-node and distant metastases [Citation7]. Yet, in this study, we found a high serum TATI associated with an adverse prognosis and with locally advanced disease. It, thus, seems that in gastric cancer the expression of TATI may affect disease progression differently depending upon whether it circulates in the plasma or is expressed in the tumor tissue. TATI was previously studied in the sera of gastric cancer patients yielding results similar to ours, whereby a high serum level associated with locally advanced disease [Citation8]. In that study, survival was not analyzed, although a high TATI level associated with metastasis, indicative of a poor outcome among patients with high TATI levels. Furthermore, in that study, statistically significant differences in the serum TATI levels between gastric cancer patients and controls were found, which we did not find here. The discrepancy between these results may be explained by the choice of controls. We selected controls with benign disease, whereas the previous study relied on healthy individuals.
The expression of trypsinogen using gastric cancer cells was previously shown to associate with invasive growth [Citation17,Citation21]. Here, we showed in a clinical setting that a high serum TAT-2 associated with pT3–4 gastric tumors and a poor outcome, providing evidence of TAT-2’s importance in tumor invasion. Similarly, gastric cancer patients’ high serum trypsinogen levels were previously shown to associate with a linitis plastica–type cancer, known for its poor prognosis due to its extremely invasive method of growth [Citation20].
TATI and CRP both function as acute-phase reactants, whereby regulating their synthesis was described as resembling that of each other [Citation9,Citation10]. Similarly, we discovered that among gastric cancer patients an elevated CRP and a high TATI level associate with each other. Interestingly, the hazard ratios among patients with high TATI or TAT-2 levels compared to those with low TATI or TAT-2 levels were higher if CRP was elevated. Thus, in patients with a systemic inflammatory response, the mechanisms via which TAT-2 and TATI may affect patient survival appear amplified.
In this study, CRP did not function as a prognostic biomarker nor did we detect differences in the CRP levels between gastric cancer patients and controls. However, similar to previous studies, we identified an elevated CRP as associated with locally advanced disease [Citation26,Citation27]. A recent meta-analysis of 2597 gastric cancer patients concluded that a high preoperative CRP level predicts a poor outcome. Yet, in 3 of the 12 original studies included in that meta-analysis, CRP failed to function as a prognostic marker, mirroring findings from our study [Citation28]. To understand why studies of CRP as a prognostic biomarker among similar gastric cancer patient cohorts with similar laboratory and statistical methodologies continue to yield conflicting results, further studies on other large, well-defined patient cohorts and further meta-analysis are necessary. We previously showed that plasma CRP, determined using similar methods, functions as a prognostic factor among pancreatic cancer patients, suggesting that the non-significance in this study does not result from an erroneous analysis [Citation24].
Whether or not these two serum markers could be applied in clinical practice in the future remains difficult to determine definitively. Perhaps patients with pT3–4 tumors and high levels of TAT-2 and TATI could benefit from more dose-intensive perioperative chemotherapy or more frequent follow-up. Furthermore, patients with a limited metastatic disease and low TAT-2 and TATI levels could potentially serve as candidates for curative surgical treatment in addition to chemotherapy. Nevertheless, these two parameters remain candidates for further exploration among patients with gastric cancer.
The strengths of this study include the large patient cohort with a long follow-up period consisting of reliable outcome and survival information. The single-center setting limits the generalizability of our findings, and several individual subgroup analyses were conducted in this study, possibly predisposing the results to familywise errors. Cutoff values calculated using Youden’s index provide high segregation performance in this patient cohort and cannot be generalized as such. Our results should be validated in independent patient cohorts, preferably in a multi-center setting. We chose not to include certain well-known risk factors for gastric cancer, such as tumor subsite, perineural and venous invasion and lymphatic emboli in the analyses, since accessing these data in a retrospective manner could introduce inaccuracies. Furthermore, we lack appropriate and detailed data on the chemotherapy administered.
In conclusion, this study shows for the first time that a high serum TAT-2 may function as a prognostic biomarker in gastric cancer and that TAT-2 levels may be elevated compared to controls. Additionally, we show that the prognosis is worse among gastric cancer patients with a high serum TATI. These biomarkers serve as prognostic factors particularly among patients with metastatic or locally advanced disease.
Supplemental Material
Download Zip (31.9 KB)Acknowledgments
The authors extend their thanks to Vanessa Fuller for exceptional English-language and style revision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Durães C, Almeida GM, Seruca R, et al. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch. 2014;464(3):367–378.
- Huhtala ML, Pesonen K, Kalkkinen N, et al. Purification and characterization of a tumor-associated trypsin inhibitor from the urine of a patient with ovarian cancer. J Biol Chem. 1982;257(22):13713–13716.
- Huhtala M-L, Kahanpää K, Seppää M, et al. Excretion of a tumor-associated trypsin inhibitor (TATI) in urine of patients with gynecological malignancy. Int J Cancer. 1983;31(6):711–714.
- Ozaki N, Ohmuraya M, Hirota M, et al. Serine protease inhibitor kazal type 1 promotes proliferation of pancreatic cancer cells through the epidermal growth factor receptor. Mol Cancer Res. 2009;7(9):1572–1581.
- Itkonen O, Stenman UH. TATI as a biomarker. Clin Chim Acta. 2014;431:260–269.
- Wiksten J-P, Lundin J, Nordling S, et al. High tissue expression of tumour-associated trypsin inhibitor (TATI) associates with a more favourable prognosis in gastric cancer. Histopathology. 2005;46(4):380–388.
- Kemik O, Kemik A, Sümer A, et al. The relationship between serum tumor-associated trypsin inhibitor levels and clinicopathological parameters in patients with gastric cancer. Eur Rev Med Pharmacol Sci. 2013;17(21):2923–2928.
- Ogawa M. Pancreatic secretory trypsin inhibitor as an acute phase reactant. Clin Biochem. 1988;21(1):19–25.
- Solakidi S, Dessypris A, Stathopoulos GP, et al. Tumour-associated trypsin inhibitor, carcinoembryonic antigen and acute-phase reactant proteins CRP and α1-antitrypsin in patients with gastrointestinal malignancies. Clin Biochem. 2004;37(1):56–60.
- Paavonen J, Lehtinen M, Lehto M, et al. Concentrations of tumor-associated trypsin inhibitor and C-reactive protein in serum in acute pelvic inflammatory disease. Clin Chem. 1989;35(5):869–871.
- Lehtovirta P, Turpeinen U, Stenman UH. Effect of intracavitary radiotherapy on tumor-associated trypsin inhibitor (TATI) in patients with cervical and endometrial cancer. Gynecol Oncol. 1990;38(1):110–113.
- Koivunen E, Huhtala ML, Stenman UH. Human ovarian tumor-associated trypsin. Its purification and characterization from mucinous cyst fluid and identification as an activator of pro-urokinase. J Biol Chem. 1989;264(24):14095–14109.
- Moilanen M, Sorsa T, Stenman M, et al. Tumor-associated trypsinogen-2 (trypsinogen-2) activates procollagenases (MMP-1, -8, -13) and stromelysin-1 (MMP-3) and degrades type I collagen. Biochemistry. 2003;42(18):5414–5420.
- Sorsa T, Salo T, Koivunen E, et al. Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem. 1997;272(34):21067–21074.
- Koshikawa N, Yasumitsu H, Umeda M, et al. Multiple secretion of matrix serine proteinases by human gastric carcinoma cell lines. Cancer Res. 1992;52 (18):5046–5053.
- Miyata S, Koshikawa N, Higashi S, et al. Expression of trypsin in human cancer cell lines and cancer tissues and its tight binding to soluble form of Alzheimer amyloid precursor protein in culture. J Biochem. 1999;125(6):1067–1076.
- Koivunen E, Saksela O, Itkonen O, et al. Human colon carcinoma, fibrosarcoma and leukemia cell lines produce tumor-associated trypsinogen. Int J Cancer. 1991;47(4):592–606.
- Stenman UH. Biomarker development, from bench to bedside. Crit Rev Clin Lab Sci. 2016;53(2):69–86.
- Ichikawa Y, Koshikawa N, Hasegawa S, et al. Marked increase of trypsin(ogen) in serum of linitis plastica (gastric cancer, borrmann 4) patients. Clin Cancer Res. 2000;6(4):1385–1388.
- Kato Y, Nagashima Y, Koshikawa N, et al. Production of trypsins by human gastric cancer cells correlates with their malignant phenotype. Eur J Cancer. 1998;34(7):1117–1123.
- Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155–170.
- Kersten C, Louhimo J, Ålgars A, et al. Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol (Madr). 2013;52(8):1691–1698.
- Salmiheimo A, Mustonen H, Stenman UH, et al. Systemic inflammatory response and elevated tumour markers predict worse survival in resectable pancreatic ductal adenocarcinoma. PLoS One. 2016;11(9):e0163064.
- Køstner AH, Kersten C, Löwenmark T, et al. The prognostic role of systemic inflammation in patients undergoing resection of colorectal liver metastases: C-reactive protein (CRP) is a strong negative prognostic biomarker. J Surg Oncol. 2016;114(7):895–899.
- Chang CC, Sun CF, Pai HJ, et al. Preoperative serum C-reactive protein and gastric cancer; clinical-pathological correlation and prognostic significance. Chang Gung Med J. 2010;33(3):301–312.
- Nozoe T, Iguchi T, Adachi E, et al. Preoperative elevation of serum C-reactive protein as an independent prognostic indicator for gastric cancer. Surg Today. 2011;41(4):510–513.
- Yu Q, Yu X-F, Zhang SD, et al. Prognostic role of C-reactive protein in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013;14(10):5735–5740.
- Kasurinen A, Tervahartiala T, Laitinen A, et al. High serum MMP-14 predicts worse survival in gastric cancer. PLoS One. 2018;13(12):e0208800.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. Hoboken (NJ): Wiley; 2011.
- Lempinen M, Isoniemi H, Mäkisalo H, et al. Enhanced detection of cholangiocarcinoma with serum trypsinogen-2 in patients with severe bile duct strictures. J Hepatol. 2007;47(5):677–683.
- Osman S, Turpeinen U, Itkonen O, et al. Optimization of a time-resolved immunofluorometric assay for tumor-associated trypsin inhibitor (TATI) using the streptavidin-biotin system. J Immunol Methods. 1993;161(1):97–106.
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35.
