Abstract
Background: To clarify local control by salvage stereotactic body radiotherapy (SBRT) for recurrent/residual hepatocellular carcinoma (HCC) compared with initial definitive SBRT for local treatment-naïve HCC.
Material and methods: We retrospectively investigated HCC patients that received SBRT between July 2005 and December 2017. We classified HCC tumors as the initial definitive SBRT group (Arm-1; initial definitive SBRT, Arm-2; initial definitive planned SBRT following transarterial chemoembolization (TACE)) and salvage SBRT group (Arm-3; salvage SBRT for recurrent/residual tumors after TACE, Arm-4; salvage SBRT for recurrent/residual tumors after radiofrequency ablation (RFA), Arm-5; salvage SBRT for recurrent/residual other than Arm-3 or Arm-4). Local control was evaluated by mRECIST.
Results: We reviewed 389 HCC tumors of 323 patients treated by 35–40 Gy/5 fr. The median follow-up time for local recurrence of tumors was 34.8 months (range, 6.5–99.2). The cumulative local recurrence rates at 3 years of Arm-1–5 were 1.4% (95% CI, 0.3–4.4%), 5.0% (95% CI, 1.6–11.5%), 12.4% (95% CI, 5.7–21.9%), 14.8% (95% CI, 3.3–34.3%) and 7.3% (95% CI, 1.9–18.0%), respectively. The cumulative local recurrence rates at 3 years of initial definitive treatment and salvage treatment groups were 2.8% (95% CI, 1.1–5.6%) and 11.1% (95% CI, 6.3–17.3%), respectively (p=.004). On multivariate analysis, salvage treatment and the tumor diameter were significant risk factors of local recurrence (p = .02, p < .001 respectively). Estimated overall survival at 3 years for all patients in initial definitive treatment and salvage treatment groups were 71.5% (95% CI, 63.4–78.1%) and 66.1% (95% CI, 56.4–74.2%), respectively (p = .20). No treatment-related death caused by SBRT was observed.
Conclusions: This analysis showed local control of salvage SBRT for recurrent/residual HCC was significantly worse than that of initial definitive SBRT for local treatment-naïve HCC. However, local control of salvage SBRT was relatively good, and salvage SBRT is one of the favorable treatment options for recurrent/residual HCC.
Introduction
Hepatocellular carcinoma (HCC) is the seventh most commonly diagnosed cancer and the fourth most common cause of cancer-related mortality worldwide [Citation1]. Curative treatments for HCC are surgery, liver transplantation and ablation such as radiofrequency ablation (RFA) according to the guidelines [Citation2,Citation3]. However, HCC patients are often contraindicated for these curative treatments because of their stage of HCC, poor general condition or technical difficulties due to the location of tumors [Citation4,Citation5]. Stereotactic body radiotherapy (SBRT) is a treatment method using high-dose radiation in a small number of fractions with a highly accurate and precise technique, and can achieve good local control for HCC [Citation6,Citation7]. In addition, SBRT can be used to treat patients with HCC located close to organs such as major vessels, bile duct or diaphragm, which are difficult to treat with RFA [Citation8]. Based on these advantages, SBRT is regarded as an acceptable option as definitive local therapy for unresectable or medically inoperable HCC [Citation9].
SBRT is often also used as salvage local therapy for recurrent/residual HCC tumors after other local therapies such as transarterial chemoembolization (TACE), RFA and surgery [Citation10–12]. However, the difference in local control of HCC between initial definitive SBRT and salvage SBRT is unclear.
The purpose of this analysis was to clarify local control after salvage SBRT for recurrent/residual HCC compared with that after initial definitive SBRT for local treatment-naïve HCC.
Material and methods
This analysis was approved by the institutional ethics board (2019-008), and all patients gave agreement with written informed consent for using their own data for future retrospective studies before treatment.
Patients
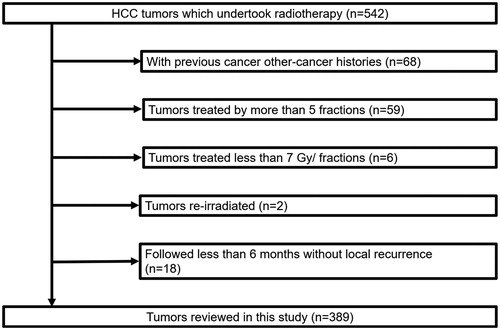
The medical records of 542 HCC tumors of 455 patients which had been treated with SBRT between July 2005 and December 2017 were extracted. We excluded patients with tumors with previous other-cancer histories, treated by more than 5 fractions, treated with less than 7 Gy per fraction, those re-irradiated due to recurrence after initial definitive SBRT, and those not followed up for more than 6 months without recurrence.
The diagnosis of HCC was mainly based on diagnostic imaging tests following the international management guidelines for HCC [Citation13]. In our institution, basic indication criteria of SBRT for HCC are as follows: (1) HCC patients who are contraindicated for surgery and RFA or refuse these treatments, (2) Child–Pugh Classification (CPC) A or B, (3) maximal diameter of tumor ≤5 cm, (4) tumor number ≤3 and (5) normal liver V20 (the volume receiving >20 Gy) not exceeding 20%. Some patients with deviation from these criteria were treated with SBRT, and those patients were included in the analysis.
Treatment
Until 2010, preceding TACE had been performed to generate visual markers for SBRT planning, but it has not been performed routinely since then.
For radiation treatment planning, patients underwent free-breathing time-averaged fast scan CT (long-time-scan CT) [Citation14,Citation15] from 6 to 8 s data acquisition/1 s time interval reconstruction in the spine position as radiotherapy-planning CT, and underwent 3- or 4-phase spiral dynamic CT using nonionic contrast medium under fixation with abdominal compression and vacuum cushions. Cone-beam CT was used before irradiation for each field to improve set-up accuracy.
Time-averaged fast scan CT and dynamic CT images were merged and gross tumor volume (GTV) was delineated on the time-averaged fast scan CT image along the enhanced lesion of the dynamic CT image in the arterial (30 s) or portal (60 s) phase, whichever was clearer. GTV included the enhanced tumor and signs of prior treatment: lipiodol deposition after TACE and low-density areas after RFA. Clinical target volume (CTV) was equal to GTV. Internal target volume (ITV) margin beyond CTV was as follows: lateral and posterior: 3 mm, anterior: 2 mm, cranial: 3–5 mm, caudal: 5–10 mm. Planning target volume (PTV) margin beyond ITV was 5 mm (cranial and caudal: 6 mm).
Radiation treatment planning systems were used to plan multi-arc dynamic conformal radiation (FOCUS XiO, version 4.2.0-4.3.3: Computerized Medical Systems, St Louis, MO) or non-coplanar volumetric-modulated arc therapy (version 10.0; Varian Medical Systems, Inc., Palo Alto, CA, USA) with the Acuros XB algorithm for heterogeneity correction. Linear accelerators (Varian Medical Systems, Inc., Palo Alto, CA, USA) were used to deliver 6 or 10-MV photons to patients.
Patients with CPC-A were treated with 40 Gy/5 fractions (fr) over 5–9 days. On the other hand, patients with CPC-B or patients whose normal liver V20 exceeded 20% with 40 Gy/5 fr regimen were treated with 35 Gy/5 fr. The total dose was prescribed as 60–80% isodose of maximum dose to cover 95% of the PTV with the prescribed dose.
Follow-up
Patients were followed up at 1 month after SBRT and every 3 months thereafter. Blood tests and diagnostic dynamic CT/MRI were performed 1 month after SBRT and then every three months. Local control was defined as no local progression within PTV evaluated by mRECIST [Citation16].
Treatment group classification
Patient groups of SBRT for HCC were classified as follows:
Initial definitive treatment group
Arm-1: Initial definitive SBRT alone
Arm-2: Initial definitive planned SBRT following TACE (documents which described clinical decision to do planned combined treatment were confirmed in chart review)
Salvage treatment group
Arm-3: Salvage SBRT for recurrent/residual tumors after TACE
Arm-4: Salvage SBRT for recurrent/residual tumors after RFA
Arm-5: Salvage SBRT for recurrent/residual other than Arm-3 or Arm-4 (e.g., after both TACE and RFA, after surgery)
Statistical analysis
To compare baseline tumors characteristics between initial definitive treatment and salvage treatment groups, t test was used for continuous variables and chi-squared test was used for categorical variables.
Time to local recurrence was defined as the time from the start of SBRT to the date of local recurrence or that of censoring. Local recurrence was estimated by cumulative incidence method considering death without local recurrence as a competing risk. Fine-Gray model analysis was performed for uni- and multivariate analyses to identify the risk factors of local recurrence. Multivariate analysis was performed for all factors with p < .20 in univariate analysis. Overall survival time was defined as the time from the start of the initial SBRT to the date of death or that of censoring. Overall survival was estimated by the Kaplan–Meier Method. Treatment related death caused by SBRT was defined as death from hepatic failure within 6 months from the start of the SBRT following the development of grade 3 liver toxicities.
Statistical analyses were carried out with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [Citation17].
Results
In this analysis, 389 HCC tumors of 323 patients were selected (). The numbers of HCC tumors in the initial definitive treatment group and salvage treatment group were 245 (Arm-1: 164, Arm-2: 81) and 144 (Arm-3: 79, Arm-4: 24, Arm-5: 41), respectively. The tumors characteristics are summarized in . The diameter of 2 and 1 tumors in the initial definitive treatment group and salvage treatment group and was more than 5 cm, respectively.
Table 1. Tumors characteristics (n = 389).
Local control
The median follow-up time for local recurrence of censored tumors was 34.8 months (range, 6.5–99.2). During the follow-up period, 21 local recurrences occurred in total (seven in initial definitive treatment group; 14 in salvage treatment group).
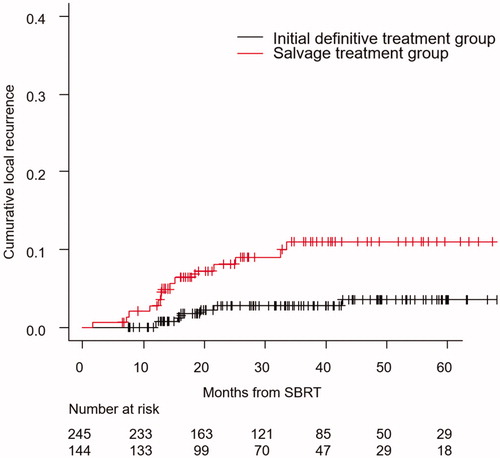
The cumulative local recurrence rates at 3 years of Arm-1–5 were 1.4% (95% CI, 0.3–4.4%), 5.0% (95% CI, 1.6–11.5%), 12.4% (95% CI, 5.7–21.9%), 14.8% (95% CI, 3.3–34.3%) and 7.3% (95% CI, 1.9–18.0%), respectively. Those in the initial definitive treatment and salvage treatment groups were 2.8% (95% CI, 1.1–5.6%) and 11.1% (95% CI, 6.3–17.3%), respectively (p = .004) ().
Figure 2. Cumulative local recurrence curve of the initial definitive treatment group/salvage treatment group.
In the univariate analysis, salvage treatment and the tumor diameter were significant risk factor for local recurrence (p = .005, p < .001, respectively), and these two factors remained as significant risk factors in multivariate analysis (p = .02, p < .001, respectively) ().
Table 2. Univariate and multivariate analyses of local recurrence.
Survival
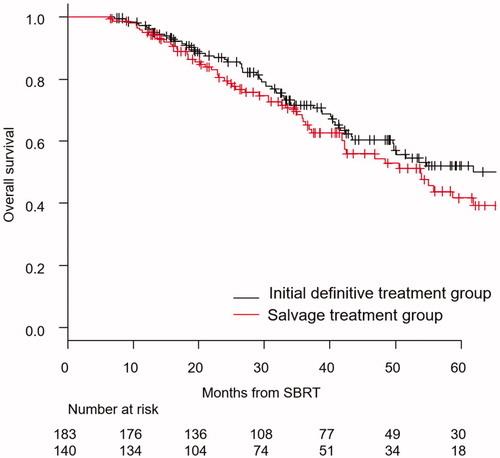
The median follow-up time for overall survival of censored patients was 37.1 months (range, 6.5–99.2). The Kaplan–Meier curves of overall survival for patients in the initial definitive treatment group/salvage treatment group are shown in . Estimated overall survival at 3 years for all patients was 69.2% (95% CI, 63.1–74.4%). Those in the initial definitive treatment and salvage treatment groups were 71.5% (95% CI, 63.4–78.1%) and 66.1% (95% CI, 56.4–74.2%), respectively (p = .20).
Toxicity
No classical radiation-induced liver disease [Citation18] was observed. No liver failure death was observed during the first 6 months (no treatment-related death caused by SBRT was observed). Between 6 and 12 months from the start of SBRT, 3 and 2 deaths from progression of liver cirrhosis were observed in the initial definitive and salvage SBRT group, respectively. No biliary or gastroenterological toxicities (≥grade 3) were observed.
Discussion
This analysis showed that the local control by salvage SBRT for recurrent/residual HCC was significantly worse than that by initial definitive SBRT for local treatment-naïve HCC, and the tumor diameter was also a significant risk factor. To our best knowledge, this is the first analysis to compare local control of HCC between initial definitive SBRT and salvage SBRT in a large number of patients.
The significantly inferior local control by salvage SBRT for recurrent/residual HCC compared with initial definitive SBRT may be explained by tumor malignancy of locally recurrent/residual HCC. Previous studies suggested that locally recurrent/residual HCC after local therapy such as TACE and RFA exhibits more biological and morphological malignant behavior than primary HCC [Citation19–22]. Imai et al. reported that locally recurrent HCC after local ablation therapy had portal vein invasion and fibrous capsule infiltration more frequently than primary HCC based on their histopathological examination for specimens of salvage hepatectomy [Citation19]. Mima et al. reported that locally recurrent HCC after local ablation therapy expressed CD44s, which has an important role in inducing expression of epithelial–mesenchymal transition [Citation23] and contributes to the metastasis and invasion of HCC [Citation24–28], higher than primary HCC. [Citation20]. Several studies suggested that hypoxia of HCC induced by TACE increase the expression of markers such as cytokeratin (CK) 19, epithelial cell adhesion molecule (EpCAM) and CD133, with all of them related to the aggressive behavior of tumors [Citation21,Citation22].
Although salvage treatment was an independent risk factor of local recurrence of HCC after SBRT in this analysis, the present results in this analysis suggest that salvage SBRT is a favorable treatment option for recurrent/residual HCC after other local treatments; cumulative local recurrence rate in salvage treatment group at 3 years was rather low at 11.1% (95% CI, 6.3–17.3%). This result was relatively better than those of salvage RFA in previous retrospective studies [Citation19,Citation29]. Imai et al. retrospectively reported local recurrence rate of salvage RFA after RFA or PEI (percutaneous ethanol injection) was 25.9% within mean 32.3 months follow-up [Citation19]. Lam et al. retrospectively reported that local recurrence of salvage RFA after TACE was 17.2% with median 24 months follow-up [Citation29]. Furthermore, Pan et al. [Citation30] retrospectively reported salvage SBRT for residual HCC after RFA showed significantly lower local disease progression rate than salvage RFA (18% vs. 59%, respectively, p=.002). Future prospective studies are warranted to compare RFA and SBRT as salvage treatments for recurrent/residual HCC.
Tumor diameter was also an independent risk factor of local recurrence of HCC after SBRT in this analysis. This result is consistent with a meta-analysis reported by Rim et al. [Citation6].
It is controversial whether dose-escalation of SBRT improves local control of HCC [Citation6,Citation7]. This analysis did not show any significant difference in local control between 35 Gy/5 fr regimen and 40 Gy/5 fr regimen. On the other hand, several studies and systematic reviews showed dose-escalation of SBRT improved local control for liver metastasis [Citation7,Citation31–35], which has generally more aggressive and radioresistant features than HCC [Citation7,Citation36]. These results of SBRT for liver metastasis suggest that dose-escalated SBRT with more than 40 Gy/5 fr in acceptable normal tissue dose constraints may improve local control of HCC with high risk factors of local recurrence such as recurrent/residual tumors and tumors with large diameter.
Although no significant difference was found in estimated overall survival at 3 years between the initial definitive treatment and salvage treatment groups, we think that it is difficult to obtain definitive knowledge from the results. This may suggest that salvage SBRT does not have a major negative impact on overall survival. To investigate overall survival after salvage SBRT, it is necessary to compare the results of salvage SBRT with other salvage therapies.
Although this analysis has strong points such as large numbers of tumors/patients and a sufficient follow-up period to evaluate 3-year local control/overall survival, there are also several limitations. This was a retrospective analysis conducted in a single institution. The number of local recurrence events in this analysis was limited. However, we did multivariate analysis appropriately with 21 (>20) local recurrence events for two independent variables; treatment group classification and tumor diameter. To our knowledge, this analysis reports the largest number of local recurrence events of HCC after SBRT [Citation36–43], and we believe the result of this analysis is valuable. There is no established method to diagnose local recurrence after radiotherapy for HCC [Citation44]. However, we used mRECIST to diagnose local recurrence as an acceptable method [Citation36,Citation44,Citation45].
Conclusions
This analysis showed that the local control of salvage SBRT for recurrent/residual HCC was significantly lower than that of initial definitive SBRT for local treatment-naïve HCC. However, local control of salvage SBRT was relatively good, and salvage SBRT is one of the favorable treatment options for recurrent/residual HCC. Dose-escalated SBRT more than 40 Gy/5 fr may improve local control of recurrent/residual HCC.
Disclosure statement
Dr. Takeda reports grants from Varian Research Grant, grants from Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science, outside the submitted work. Other authors declare no conflicts of interest.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750.
- Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236.
- Liu JH, Chen PW, Asch SM, et al. Surgery for hepatocellular carcinoma: does it improve survival? Ann Surg Oncol. 2004;11(3):298–303.
- Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144.
- Ohri N, Tome WA, Mendez Romero A, et al. Local control after stereotactic body radiation therapy for liver tumors. Int J Radiat Oncol Biol Phys. 2018.
- Ohri N, Dawson LA, Krishnan S, et al. Radiotherapy for hepatocellular carcinoma: new indications and directions for future study. J Natl Cancer Inst. 2016;108(9).
- Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 1.2017: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2017;15(5):563–573.
- Buckstein M, Kim E, Fischman A, et al. Stereotactic body radiation therapy following transarterial chemoembolization for unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2018;9(4):734–740.
- Honda Y, Kimura T, Aikata H, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28(3):530–536.
- Kang JK, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118(21):5424–5431.
- Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122–137.
- Mori S, Kanematsu N, Mizuno H, et al. Physical evaluation of CT scan methods for radiation therapy planning: comparison of fast, slow and gating scan using the 256-detector row CT scanner. Phys Med Biol. 2006;51(3):587–600.
- Takeda A, Kunieda E, Shigematsu N, et al. Small lung tumors: long-scan-time CT for planning of hypofractionated stereotactic radiation therapy – initial findings. Radiology. 2005;237(1):295–300.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458.
- Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3):S94–S100.
- Imai K, Beppu T, Chikamoto A, et al. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol Res. 2014;44(14):E335–E345.
- Mima K, Hayashi H, Imai K, et al. High CD44s expression is associated with the EMT expression profile and intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepato-Biliary-Pancreatic Sci. 2013;20(4):429–434.
- Lai JP, Conley A, Knudsen BS, et al. Hypoxia after transarterial chemoembolization may trigger a progenitor cell phenotype in hepatocellular carcinoma. Histopathology. 2015;67(4):442–450.
- Nahm JH, Rhee H, Kim H, et al. Increased expression of stemness markers and altered tumor stroma in hepatocellular carcinoma under TACE-induced hypoxia: a biopsy and resection matched study. Oncotarget. 2017;8(59):99359–99371.
- Mima K, Okabe H, Ishimoto T, et al. CD44s regulates the TGF-beta-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. 2012;72(13):3414–3423.
- Chen L, Chan TH, Yuan YF, et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J Clin Invest. 2010;120(4):1178–1191.
- Lee TK, Poon RT, Yuen AP, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial–mesenchymal transition. Clin Cancer Res. 2006;12(18):5369–5376.
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273.
- Thiery JP, Acloque H, Huang RY, et al. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890.
- Yang MH, Chen CL, Chau GY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50(5):1464–1474.
- Lam VW, Ng KK, Chok KS, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207(1):20–29.
- Pan YX, Xi M, Fu YZ, et al. Stereotactic body radiotherapy as a salvage therapy after incomplete radiofrequency ablation for hepatocellular carcinoma: a retrospective propensity score matching study. Cancers (Basel). 2019;11(8):1116.
- Takeda A, Sanuki N, Tsurugai Y, et al. Stereotactic body radiotherapy for patients with oligometastases from colorectal cancer: risk-adapted dose prescription with a maximum dose of 83–100 Gy in five fractions. J Radiat Res. 2016;57(4):400–405.
- Andratschke N, Alheid H, Allgauer M, et al. The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. BMC Cancer. 2018;18(1):283.
- Joo JH, Park JH, Kim JC, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017;99(4):876–883.
- Mahadevan A, Blanck O, Lanciano R, et al. Stereotactic body radiotherapy (SBRT) for liver metastasis – clinical outcomes from the international multi-institutional RSSearch(R) Patient Registry. Radiat Oncol. 2018;13(1):26.
- Petrelli F, Comito T, Barni S, et al. Stereotactic body radiotherapy for colorectal cancer liver metastases: a systematic review. Radiother Oncol. 2018;129(3):427–434.
- Yamashita H, Onishi H, Matsumoto Y, et al. Local effect of stereotactic body radiotherapy for primary and metastatic liver tumors in 130 Japanese patients. Radiat Oncol. 2014;9(1):112.
- Uemoto K, Doi H, Shiomi H, et al. Clinical assessment of micro-residual tumors during stereotactic body radiation therapy for hepatocellular carcinoma. Anticancer Res. 2018;38(2):945–954.
- Kuo HT, Que J, Lin LC, et al. Impact of tumor size on outcome after stereotactic body radiation therapy for inoperable hepatocellular carcinoma. Medicine (Baltimore). 2017;96(50):e9249.
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34(5):452–459.
- Scorsetti M, Comito T, Cozzi L, et al. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol. 2015;141(7):1301–1309.
- Bibault JE, Dewas S, Vautravers-Dewas C, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS One. 2013;8(10):e77472.
- Jang WI, Kim MS, Bae SH, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8(1):250.
- Jackson WC, Suresh K, Maurino C, et al. A mid-treatment break and reassessment maintains tumor control and reduces toxicity in patients with hepatocellular carcinoma treated with stereotactic body radiation therapy. Radiother Oncol. 2019;141:101–107.
- Schaub SK, Hartvigson PE, Lock MI, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: current trends and controversies. Technol Cancer Res Treat. 2018;17.
- Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53(3):399–404.