Abstract
Background: A prospective study on shoulder and arm morbidity was conducted in Denmark in 2003–2005. This study demonstrated that sentinel lymph node biopsy was associated with better outcomes than axillary lymph node dissection 18 months after surgery. We here aimed to describe subjective symptoms and objective findings in these patients 10+ years after they underwent breast cancer surgery and to assess how symptoms and findings developed during this period.
Material and methods: Participants in the prospective study completed a questionnaire and underwent an objective, bilateral examination of their shoulder and arm morbidity, which included measurement of arm volume, range of motion, and sensibility.
Results: Seventy participants completed follow-up. Thirty-four (49%) had one or more functional impairments, and 64% had one or more subjective loco regional symptoms like pain, swelling of the arm, and decreased shoulder mobility. Objective evaluation showed 34 ml’s of increased arm volumes and 3–25% had severe reduced shoulder mobility on the operated side. Compared to the findings at 18 months postoperatively, small but significant differences in occurrence of subjective findings were observed. A significant progression regarding most objective findings was revealed.
Conclusion: More than 11 years after breast cancer surgery, the majority of participants complained of one or more subjective symptoms of shoulder and arm morbidity. Objective findings were mild or modest in most cases. During the prolonged follow-up period of 10 years, a worsening in symptoms and objective findings was observed.
Shoulder and arm morbidity in relation to breast cancer treatment seems to progress beyond 10 years.
The most frequent symptoms were pain, swelling or heaviness of the arm, and decreased shoulder mobility.
The objective evaluation showed higher arm volumes and reduced shoulder mobility on the operated side.
Objective findings are mild and modest but may affect activities of daily living, and most participants with late symptoms stated that this was a daily problem.
HIGHLIGHTS
Introduction
During the past few decades, overall survival from breast cancer (BC) has improved and late morbidity following treatment has attracted growing attention. A follow-up study conducted by the Danish Breast Cancer Cooperative Group (DBCG) from 2003 to 2005 focused on objective and subjective arm morbidity and discovered less morbidity among lymph node negative patients who had sentinel lymph node biopsy (SLNB) than among patients who had axillary lymph node dissection (ALND) [Citation1]. Breast-conserving surgery (BCS) has also been shown to be more advantageous than modified radical mastectomy in relation to degree of shoulder disability [Citation2]. Only few studies on shoulder morbidity [Citation3–5] but several studies on arm lymphedema (ALE) [Citation6–10] have followed patients over a longer period and supported the findings that less extensive surgery reduces the risk of loco regional shoulder and arm morbidity.
A systematic review suggests that one in five survivors from BC will develop ALE [Citation6]. Debate about the various definitions and methods of measuring ALE is ongoing [Citation11–14]. Using questionnaires, a cohort study showed that 13–65% of patients with BC reported a feeling of swelling of the arm, and that this symptom was associated with ALND, young age, and radiation therapy (RT) [Citation9]. Persistent pain was experienced by 22–53% of women after BC treatment; and an association with BCS, ALND, adjuvant chemotherapy, and RT was found [Citation15]. A study compared patients with BC with the general population and found a higher prevalence of chronic pain among survivors from BC (42%) [Citation16]. ALND has also been found to be associated with the risk of developing measurable ALE [Citation6,Citation17,Citation18] and restricted shoulder movement [Citation1].
The correlation between subjective symptoms and objective findings regarding ALE has shown little consistency; suggesting shortcomings in screening tools and questionnaires used for investigating different aspects of lymphedema or that subjective symptoms may represent a subclinical stage of ALE [Citation7,Citation8].
Most studies of late morbidity related to BC treatment cover a period of less than 5 years. Some retrospective long-term follow-up (LT-FU) studies have been made [Citation19–21], but no prospective LT-FU studies have come to our attention.
We here aim to describe subjective symptoms and objective findings of shoulder and arm morbidity in patients who participated in the previous DBCG study 10+ years after they underwent surgery and to assess how symptoms and findings developed during this period [Citation1]. The participants were evaluated using the same questionnaire and objective measures as those used when they completed the DBCG study [Citation1]. This gave us the opportunity to compare data recorded preoperatively and at 18 months after surgery with our newly collected LT-FU data, and hence to elucidate changes in the 10 years from the first follow-up at 18 months to the present at 10+ years.
Material and methods
Study design
A prospective follow-up study including subjective and objective evaluation at 12 years after BC surgery including data from baseline and 18 months after surgery. The study was planned and conducted according to the EQUATOR guidelines (Supplementary 1) [Citation22].
Study population
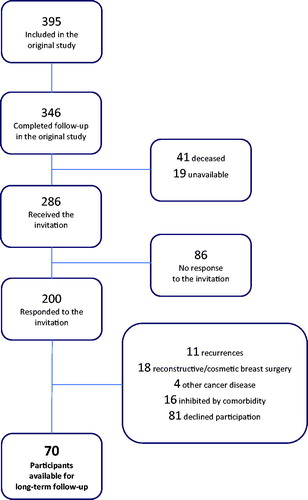
As a part of the implementation of SLNB in Denmark in 2003–2004, a study population of 395 women with unifocal, early BC was recruited by Husted Madsen et al. [Citation1]. The study included follow-up by physical examination and questionnaire, preoperatively and at 18 months after surgery, which was completed by 346 participants ().
Survival data were extracted from the national Danish Central Personal Register (CPR). Of 286 women available for the present study who invited to participate by letter, 200 (70%) responded, some of whom were unable to participate. Eighty-one women declined participation. Left for the present LT-FU study were 70 (24%) participants. They completed a physical examination and a questionnaire during the period May 2015–April 2016. Fifty-seven participants were evaluated preoperatively; 63 at the 6-month follow-up, 65 at the 18 months follow-up; 70 at the 10+ years LT-FU; 50 participants completed all of the four follow-up sessions.
Subjective evaluation
The questionnaire from the original study was reused. It comprised questions regarding pain, sensibility, swelling of the arm, and restriction of shoulder movement. Questions about functional impairments were added. If a symptom was present, the participant was asked to grade the inconvenience by a ‘Bother Score’ (BS) (1 = no problem, 2 = minor problem, 3 = moderate problem, 4 = major problem). In case of pain, we used a supplement with a visual analog scale (VAS) graded from 1 to 10.
The original questionnaire [Citation1] was tested in a pilot study prior to the present study by a small group of patients with BC operated 18 months previously. Only minor changes were made to ease understanding of the questionnaire.
During data analysis, subjective symptoms were extracted from the questionnaires. Most were dichotomous (Yes/No) and some were dichotomized during data analysis.
Objective evaluation
The participants had a thorough examination of the breast region and both shoulders and arms. Findings are listed as ‘operated’, referring to the same side of the BC operation, and ‘non-operated’, referring to the contralateral side. ‘Difference’ is the absolute difference between the two sides.
The physical examinations at LT-FU were the same as in the original study, albeit we did choose to use the Simplified Water Displacement Instrument (SWDI) [Citation23,Citation24] owing to its simplicity instead of the more traditional water displacement volumetry. At the test of passive abduction, we chose to focus on the isolated gleno-humeral range of motion (ROM) instead of abduction combined with scapular thoracic motion.
Preoperatively, available measurements from 57 participants showed a mean difference in arm volume of 36.6 ml (2.1%) in favor of the dominant arm. This preoperative difference was highly significant (p < .01), which was compensated for by individual adjustment to calculate the ‘adjusted difference’ in arm volumes at LT-FU by subtraction of the preoperative difference from the arm volume of the dominant arm at LT-FU [Citation23,Citation25].
Considering the normal anatomical ROM and the mobility required for activities of daily living (ADL) [Citation26], we defined ranges of mobility restriction as ‘severe’ (restriction may affect ADL), ‘moderate’ (only minor measurable limitation), and ‘none’ (no or mild mobility restriction). These ranges were used to group the participants and consider the prevalence.
Data collection
Following approval from The Local Research Ethics Committee of the Central Region Denmark and The Danish Data Protection Agency, we obtained data from the CPR and the original study [Citation1]. Whenever data were missing, the DBCG database was searched for missing data. Study data were collected and managed using the REDCap electronic data capture tools hosted at Aarhus University, Denmark [Citation27].
Analysis and statistics
To estimate the presence of a subjective symptom, we determined the prevalence and prevalence proportion. The majority of the objective data showed normal distribution, and mean values with 95% confidence intervals (CIs) were calculated and shown with ranges. Comparison of the prevalence of symptoms and dichotomous, objective findings between operation groups was made with Fisher’s exact test. A comparison of mean values between the operation groups was made with one-way analysis of variance (ANOVA). The development in symptoms and findings over time was assessed by Fisher’s exact test.
The statistical software package Stata/IC 13 (StataCorp LLC) was used for the analysis. The significance level was set at p < .05 (two-sided tests).
Results
The characteristics of the 70 participants and their BC treatment are shown in . The median follow-up time at LT-FU was 11.7 years (range: 11.0–12.5) at which time the median age was 64.8 years (range: 51.5–78.5).
Table 1. Patient, tumor, and treatment characteristics.
The mean Body Mass Index (BMI) at LT-FU was 24.6. BMI was found to be rather stable: 24.5 preoperatively and 25.1 at 18 months of follow-up.
BCS had been performed on 49 (70%) and mastectomy on 21 (30%) participants. They all had axillary surgery of whom 25 (35.7%) had SLNB and 45 (64.3%) had ALND. Split into operation groups, the participants were distributed as follows: Group 1: BCS + SLNB (n = 22); Group 2: BCS + ALND (n = 27); Group 3: Mastectomy + SLNB (n = 3); Group 4: Mastectomy + ALND (n = 18).
Every participant who had BCS had received RT. Among patients having undergone mastectomy, those with lymph node metastasis had received RT. In total, 87% of the participants had received RT.
No significant differences in participant and treatment characteristics were found between the larger original study cohort and the smaller cohort at LT-FU – except for the number of smokers before their operation (34% in the original study cohort and 20% in the cohort at LT-FU).
Subjective symptoms
The main findings from the questionnaire evaluation are shown in . Thirty-four participants (49%) reported one or more changes in functional impairment, with a prevalence of the individual symptoms between 10% and 41%. Decreased hand and arm functioning was reported by 24%, and 41% had changed their habits of carrying heavy bags. Swelling of the arm was reported by 26% and was a minor problem (BS = 2) to the majority. Five of them were using a compression sleeve. Decreased shoulder mobility was reported by 26%, and was scored BS = 1–2 by more than half. It was noted that 57% were doing mobility exercises weekly or frequently. Some 30% and 53% stated pain in the shoulder or operated breast region, respectively. The pain intensity graded by VAS revealed a median of 4 in the shoulder. VAS was higher among mastectomized participants than among those who had BCS. Comparing the prevalence of each late symptom between the operation groups, we found no significant differences. Some 64% of the participants complained of one or more subjective symptoms.
Table 2. Questionnaire evaluation.
We compared participants’ subjective symptoms at LT-FU to what was reported at their latest prior follow-up (18 months postoperatively) to determine developments in symptoms over time (). At 18 months of follow-up, 40% of the participants stated pain in the operated area, which increased to 52% at LT-FU. Progression in shoulder pain was less obvious. Hand and arm function improved. Thus, 39% reported affected hand and arm function at 18 months compared with 24% at LT-FU. Over time, fewer participants reported swelling of the arm (35% versus 26%), but the use of compression sleeve had increased from 1 participant at the 18 months follow-up to 5 participants at LT-FU. The prevalence of most of the symptoms seemed rather stationary, although significant changes were shown.
Table 3. Changes in shoulder and arm morbidity in 10 years.
Objective examination
The most prominent findings emerging from the objective evaluation are shown in Supplementary 2 for arms and shoulders. We found an adjusted, absolute difference in arm volumes of 33.8 ml. Every passive shoulder ROM showed a minor reduction in mobility in the ‘operated’ compared with the ‘non-operated’ shoulder. No significant difference in arm volumes or reduced mobility was observed between operation groups, but individual variations were large.
Using the definition of ALE as a > 10% difference in arm volumes, we found that only four participants (8%) met the criteria of ALE (); a result that differs from that obtained at 18 months. This comparison indicates a slight but significant (p = .019) progression in the ALE grade over time.
When mobility restriction was defined as ‘moderate’ to ‘severe’, 3–25% showed a ‘severe’ reduction in ROM that might affect ADL. Comparing changes from 1.5 years of follow-up to LT-FU (), we found a tendency toward worsening in the shoulder ROM for the ‘operated’ shoulder in all the directions. A significant difference was found for flexion and outward rotation.
Sensibility changes of the upper arm were seen among 43% (Supplementary 2), and they were more strongly associated with ALND than with SLNB (p < .05) but not with breast surgery. From 18 months follow-up until LT-FU, 12 participants recovered from their sensibility disturbance of the upper arm (), and this improvement was significant.
Discussion
To our knowledge, the present study is the first to span more than 10 years of follow-up on both subjective and objective shoulder and arm morbidity issues following BC treatment. We studied development in shoulder and arm morbidity over time.
The prevalence of pain in the operated area was 53%, and those who were most affected by pain had had BCS. Our findings confirm the tendency reported by Peuckmann et al. [Citation16]. The present study also shows a high prevalence proportion of pain in the shoulder (30%) or arm (21%). This finding also correlates with findings reported in other studies [Citation28]. Adjuvant chemotherapy is also considered causing late morbidity as pain and paresthesia. Others have focused on this topic and found persistent pain associated to the adjuvant chemotherapy [Citation29].
Comparison with previous studies is hampered by the fact that definitions of ALE differ across studies. Some compare the ‘operated’ and the ‘non-operated’ arms and define ALE as a > 2 cm difference in upper arm circumference, a > 200 ml difference according to volumetry, or a > 10% difference in circumference or volume. Studies including preoperative evaluations frequently use the same definitions but compare the ‘operated’ arm before and after BC treatment.
We included preoperative information on natural arm volume differences to calculate adjusted arm volumes. Others before us have commented on the importance of considering the natural asymmetry between the dominant and non-dominant arm [Citation23,Citation25]. Even so, we are not familiar with any recent studies in which arm volumes have been adjusted according to preoperative differences as done here. Arm volumes and degrees of ALE on the ‘operated’ arm increased in the 10- year period since the last follow-up. This has also been shown in other studies, which, however, all had a shorter follow-up period than the present study [Citation3,Citation7,Citation30].
Both RT and axillary surgery are known to affect the risk of ALE [Citation6,Citation10,Citation31,Citation32]. A large majority (87%) of our participants had received RT. It may be considered a weakness of our study that we do not report on various aspects of RT as it is known that adjuvant RT may impact the results. However, we are here concerned with the overall radiation effect for which purpose no such details was deemed necessary. The AMAROS trial compared ALND with axillary RT; at 5 years, the risk of ALE was 15% after axillary RT alone and 25% after ALND alone [Citation10]. In studies focusing on the risk of ALE associated with the extent of axillary surgery, SLNB has been found to carry a lower risk than ALND [Citation6,Citation31]. Also, the number of removed lymph nodes has been found to influence the risk of ALE [Citation6,Citation33]. The risk of ALE was increased after ALND in both node positive and node negative patients compared to after SLNB as shown by Sackey et al. [Citation34]. Currently, the SENOMAC randomized trial (NCT 02240472, www.clinicaltrials.gov) investigates the consequences of refraining from ALND in BC patients with 1–2 sentinel nodes with macrometastasis (www.senomac.se). Two thirds of the participants in the present study had ALND, but we found no significant difference in the prevalence of ALE between the operation groups. The risk of ALE has also been associated with increased BMI [Citation35] and young age [Citation36]. The 10-year increase in arm volumes could have been explained by increasing BMI with age, but we observed a rather stable BMI.
Our findings of decreased mobility in the ‘operated’ shoulder did not appear to be associated with the type of surgical treatment. Others have found decreased mobility to be associated with RT, ALND, and mastectomy [Citation2,Citation4,Citation32,Citation37,Citation38]. According to the questionnaire, 57% of our study population was still doing exercises more than twice weekly to maintain shoulder mobility. This might have biased our results. The mobility might have been further decreased if they were not doing exercises. Axillary web syndrome (AWS) is also associated with decreased mobility and the occurrence of pain [Citation39,Citation40]. In the present study, AWS was not investigated as a specific endpoint, albeit no AWS was noticed at the LT-FU or reported at the 18 months follow up.
In the present study, abduction was compromised over time on the operated side. However, reviewing the examination technique at LT-FU, we realized that our focus on the isolated gleno-humeral motion during abduction may have differed from the focus adopted at the earlier follow-up examinations. To account for this apparent systematic error, we calculated the percentage differences between the ‘operated’ and the ‘non-operated’ arms to facilitate comparison of the LT-FU data with the 18-month follow-up data. Shoulder-arm morbidity in general and mobility in particular seem to affect both the operated and the contralateral side [Citation5,Citation41,Citation42]. At each follow-up session, our study population was evaluated bilaterally and we do have baseline values for comparison.
Sensibility of the upper arms improved from 18 months to LT-FU. This suggests that the improvement may partly be caused by reversible neuropraxia and not by complete nerve lesion.
It may be a weakness of the present study that although it has been used in previous studies, the questionnaire has never been validated. Some of the questions about ADL used in the LT-FU setting were intentionally picked from a validated questionnaire [Citation9]. These questions were piloted and evaluated for relevance and comprehensibility before baseline and before the LT-FU. However, to enhance the quality of the questionnaire, a validation study is demanded.
The present study was limited by a rather small size of the study cohort. A larger cohort might have given a clearer picture. The small study population was represented by a group of relatively younger (median age = 54 years at diagnosis) and less frequently smoking women (). In Denmark, the median age at diagnosis of BC is 62 years. Furthermore, some of the invited women declined participation due to comorbidity (N = 16). These negative responders might have more pronounced symptoms and findings of shoulder and arm morbidity. We do not possess knowledge of the reason why non-responders (N = 86) did not answer to the invitation. The frequencies and extent of symptoms and findings may be biased due to this selection of participants, which complicates generalization from our study. Albeit, our focus on the development in symptoms and findings in the available study population is not considered to be affected by this bias.
Registration of Patient Reported Outcome Measures (PROM) might improve the knowledge on late effects of breast cancer treatment. Actually, a very ambitious PROM-study is underway in Denmark. In that study, patients will from diagnosis of BC and throughout treatment and follow-up repeatedly be asked to report on symptoms via an interactive application using computer-adaptive testing (CAT). Every patient with newly diagnosed early BC will be asked to participate.
The late symptoms related to BC treatment may change over time, becoming either better or worse, as shown by others [Citation3,Citation7]. Therefore, baseline evaluations and repeated follow-up are important, as illustrated in the present study. Our findings of symptom development over time echo findings reported in previous studies [Citation3,Citation7]. However, the present LT-FU study also shows that some symptoms progress even beyond 5 years, indeed up to 11.5 years. Although our study population consists of relatively young patients with BC with a median age of 64.8 (range 51.5–78.5) at LT-FU, we cannot ignore the possibility that some of the symptoms or objective findings may be related not to BC treatment per se but to increasing age.
Conclusion
More than 11 years after BC treatment, two out of three participants complained of one or more subjective symptoms of shoulder and arm morbidity. The objective findings were in most cases mild or modest but might affect ADL.
During the prolonged follow-up period of 10+ years, a worsening in symptoms and objective findings was observed.
Trial registration
ClinicalTrial.gov, registration number: NCT02159274 (2014).
The Local Research Ethics Committee for Central Region Denmark: 1-16-02-669-14 (2014) and 1-10-72-335-13 (2013).
| Abbreviations | ||
| ADL | = | activities of daily living |
| ALE | = | arm lymphedema |
| ALND | = | axillary lymph node dissection |
| ANOVA | = | one-way analysis of variance |
| AWS | = | axillary web syndrome |
| BC | = | breast cancer |
| BCS | = | breast-conserving surgery |
| BMI | = | Body Mass Index |
| BS | = | Bother Score |
| CI | = | confidence interval |
| CPR | = | Central Personal Register |
| DBCG | = | Danish Breast Cancer Cooperative Group |
| LT-FU | = | long-term follow-up |
| Mast. | = | mastectomy |
| PROM | = | patient reported outcome measures |
| ROM | = | range of motion |
| RT | = | radiation therapy |
| SLNB | = | sentinel lymph node biopsy |
| SWDI | = | simplified water displacement instrument |
| VAS | = | visual analog scale |
Supplemental Material
Download MS Word (19.6 KB)Supplemental Material
Download MS Word (30.4 KB)Acknowledgments
The authors also thank the staff at the Breast Clinic, Department of Plastic and Breast Surgery, Aarhus University Hospital, the medical students assisting our research, and first and foremost the study participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Husted Madsen A, Haugaard K, Soerensen J, et al. Arm morbidity following sentinel lymph node biopsy or axillary lymph node dissection: a study from the Danish Breast Cancer Cooperative Group. Breast. 2008;17(2):138–147.
- Lauridsen MC, Overgaard M, Overgaard J, et al. Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 2008;47:569–575.
- Sagen A, Kåresen R, Sandvik L, et al. Changes in arm morbidities and health-related quality of life after breast cancer surgery – a five-year follow-up study. Acta Oncol. 2009;48(8):1111–1118.
- Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treatment. 2009; 116(1):1–15.
- Adriaenssens N, Vinh-Hung V, Miedema G, et al. Early contralateral shoulder-arm morbidity in breast cancer patients enrolled in a randomized trial of post-surgery radiation therapy. Breast Cancer (Auckl). 2012;6:79–93.
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515.
- Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology 2010;43(3):118–127.
- Bulley C, Gaal S, Coutts F, et al. Comparison of breast cancer-related lymphedema (Upper Limb Swelling) prevalence estimated using objective and subjective criteria and relationship with quality of life. Biomed Res Int. 2013;2013:1–8.
- Gärtner R, Jensen M-B, Kronborg L, et al. Self-reported arm-lymphedema and functional impairment after breast cancer treatment – a nationwide study of prevalence and associated factors. Breast. 2010;19(6):506–515.
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310.
- ISL. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Acta Angiol. 2017;23:171–182.
- Hayes S, Cornish B, Newman B. Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat. 2005;89(3):221–226.
- Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–217.
- McLaughlin SA, Staley Ac Vicini F, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema: recommendations from a multidisciplinary expert ASBrS Panel Part 1: definitions, assessments, education, and future directions. Ann Surg Oncol. 2017;24:2818–2826.
- Mejdahl MK, Andersen KG, Gartner R, et al. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ. 2013;346( 1):f1865–f1865.
- Peuckmann V, Ekholm O, Rasmussen NK, et al. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain. 2009;13(5):478–485.
- Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–1972.
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol. 2008;26(32):5220–5226.
- Lyngholm CD, Christiansen PM, Damsgaard TE, et al. Long-term follow-up of late morbidity, cosmetic outcome and body image after breast conserving therapy. A study from the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2013;52(2):259–269.
- Johansen J, Overgaard J, Rose C, et al. Cosmetic outcome and breast morbidity in breast-conserving treatment cancer. Acta Oncol (Madr). 2002;41:368–380.
- Johansson K, Branje E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol (Madr). 2010;49(2):166–173.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457.
- Risberg MA, Sagen A, Kåresen R. The reliability of a simplified water displacement instrument: a method for measuring arm volume. Arch Phys Med Rehabil. 2005;86(1):86–89.
- Sagen A, Kåresen R, Skaane P, et al. Validity for the simplified water displacement instrument to measure arm lymphedema as a result of breast cancer surgery. Arch Phys Med Rehabil. 2009;90(5):803–809.
- Godal R, Swedborg I. A correction for the natural asymmetry of the arms in the determination of the volume of oedema. Scand J Rehabil Med. 1982;14(4):193–195.
- Namdari S, Yagnik G, Ebaugh DD, et al. Defining functional shoulder range of motion for activities of daily living. J Shoulder Elb Surg. 2012;21(9):1177–1183.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Miaskowski C, Paul SM, Cooper B, et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. 2014;18(3):242–253.
- Tasmuth T, von Smitten K, Hietanen P, et al. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6:2024–2031.
- Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. QJM Int J Med. 2005;98(5):343–348.
- Miller CL, Specht MC, Skolny MN, et al. Risk of lymphedema after mastectomy: potential benefit of applying ACOSOG Z0011 protocol to mastectomy patients. Breast Cancer Res Treat. 2014;144:71–77.
- Johansen S, Fosså K, Nesvold IL, et al. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol. 2014;53(4):521–529.
- Goldberg JI, Wiechmann LI, Riedel ER, et al. Morbidity of sentinel node biopsy in breast cancer: the relationship between the number of excised lymph nodes and lymphedema. Ann Surg Oncol. 2010;17(12):3278–3286.
- Sackey H, Magnuson A, Sandelin K, et al. Arm lymphoedema after axillary surgery in women with invasive breast cancer. Br J Surg. 2014;101(4):390–397.
- Helyer LK, Varnic M, Le LW, et al. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16(1):48–54.
- Armer J, Fu MR. Age differences in post-breast cancer lymphedema signs and symptoms. Cancer Nurs. 2005;28(3):200–207; quiz 208–209.
- Højris I, Andersen J, Overgaard M, et al. Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic treatment versus systemic treatment alone. Acta Oncol (Madr). 2000;39(3):355–372.
- Bentzen SM, Overgaard M, Thames HD. Fractionation sensitivity of a functional endpoint: impaired shoulder movement after post-mastectomy radiotherapy. Int J Radiat Oncol. 1989;17(3):531–537.
- Yeung WM, McPhail SM, Kuys SS. A systematic review of axillary web syndrome (AWS). J Cancer Surviv. 2015;9(4):576–598.
- Koehler LA, Haddad TC, Hunter DW, et al. Axillary web syndrome following breast cancer surgery: symptoms, complications, and management strategies. BCTT. 2019;11:13–19.
- Sagen A, Kaaresen R, Sandvik L, et al. Upper limb physical function and adverse effects after breast cancer surgery: a prospective 2.5-year follow-up study and preoperative measures. Arch Phys Med Rehabil. 2014;95:875–881.
- Shamley D, Lascurain-Aguirrebeña I, Oskrochi R, et al. Shoulder morbidity after treatment for breast cancer is bilateral and greater after mastectomy. Acta Oncol. 2012;51(8):1045–1053.