Abstract
Background: Proximal esophageal cancer (EC) is commonly treated with definitive chemoradiation (CRT). The radiation dose and type of chemotherapy backbone are still under debate. The objective of this study was to compare the treatment outcomes of contemporary CRT regimens.
Material and Methods: In this retrospective observational cohort study, we included patients with locally advanced squamous cell cancer of the proximal esophagus, from 11 centers in the Netherlands, treated with definitive CRT between 2004 and 2014. Each center had a preferential CRT regimen, based on cisplatin (Cis) or carboplatin-paclitaxel (CP) combined with low (≤50.4 Gy) or high (>50.4 Gy) dose radiotherapy (RT). Differences in overall survival (OS) between CRT regimens were assessed using a fully adjusted Cox proportional hazards and propensity score (PS) weighted model. Safety profiles were compared using a multilevel logistic regression model.
Results: Two hundred patients were included. Fifty-four, 39, 95, and 12 patients were treated with Cis-low-dose RT, Cis-high-dose RT, CP-low-dose RT, and CP-high-dose RT, respectively. Median follow-up was 62.6 months (95% CI: 47.9–77.2 months). Median OS (21.9 months; 95% CI: 16.9–27.0 months) was comparable between treatment groups (logrank p = .88), confirmed in the fully adjusted and PS weighted model (p > .05). Grades 3–5 acute adverse events were less frequent in patients treated with CP-low-dose RT versus Cis-high-dose RT (OR 3.78; 95% CI: 1.31–10.87; p = .01). The occurrence of grades 3–5 late toxicities was not different between treatment groups.
Conclusion: Our study was unable to demonstrate a difference in OS between the CRT regimens, probably related to the relatively small sample size. Based on the superior safety profile, carboplatin and paclitaxel-based CRT regimens are preferred in patients with locally advanced proximal EC.
Introduction
Ten percent of all esophageal cancers (ECs) are located in the proximal part of the esophagus, which consists of the cervical and upper thoracic segment [Citation1]. Proximal EC is challenging to treat due to the vicinity of vital structures including the larynx, cricoid, and trachea, which are frequently infiltrated. Furthermore, patients with proximal EC often have locoregional lymph node metastases at the time of diagnosis [Citation2]. Most patients with proximal EC are thus being diagnosed at an unresectable stage. In others, surgical treatment would implicate mutilating resections, with a high risk of major complications and an extensive impact on a patient’s quality of life [Citation3–5].
As shown in the Radiation Therapy Oncology Group (RTOG) 85-01 trial, a nonsurgical approach of definitive chemoradiation (CRT) provides a significant survival advantage over monomodality radiotherapy (RT) in patients with EC [Citation6]. Hence, CRT is the standard treatment modality in patients with proximal EC recommended by the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) [Citation7,Citation8]. However, there are no data on the most effective CRT regimen in proximal EC [Citation9]. Hence, physicians use either established regimens for the treatment of head and neck squamous cell carcinoma (HNSCC) due to similarities in location, histology, and molecular features [Citation10], or regimens used in the management of patients with lower EC [Citation9]. The most frequently used CRT regimen for HNSCC is 66–70 Gy RT, with concurrent cisplatin (Cis) at a minimum cumulative dose of 200 mg/m2 body surface intravenously in a 7-week treatment period [Citation11,Citation12].
In EC, the radiation dose and type of chemotherapy backbone in CRT are still under debate. In the definitive CRT setting, radiation doses of 50–50.4 Gy are recommended to cancers originating in the esophagus [Citation6–8]. NCCN guidelines state that higher doses of RT (60–66 Gy) may be appropriate for cancers in the proximal esophagus, although sufficient data to substantiate this statement are lacking [Citation7].
Chemotherapy schemes containing Cis and 5-fluorouracil (5-FU) have long been the standard in CRT regimens for EC [Citation6,Citation8,Citation13]. CRT with paclitaxel plus 5-FU did not improve survival over Cis and 5-FU-based CRT [Citation14]. The CROSS trial [Citation15] demonstrated acceptable adverse event rates for carboplatin-paclitaxel (CP)-based pre-operative CRT in patients with EC, with a pathologic complete response rate of 49% in patients with squamous cell EC, which was higher than previously observed in Cis-5-FU regimens [Citation16–18]. These results have led to the use of CP in definitive CRT schedules [Citation19]. Accordingly, CRT regimens for EC with Cis or CP are recommended by the NCCN and ESMO [Citation7,Citation8]. Results from a small comparative study in the definitive treatment of EC support implementing CP-based CRT, showing comparable survival and a more favorable safety profile in patients treated with CP compared with Cis-based CRT [Citation20]. However, this study did not include cervical EC, and only a minority of patients were treated with a radiation dose exceeding 50.4 Gy. We hypothesized that patients with proximal EC treated with CP-based definitive CRT with low-dose RT have the best outcome in terms of adverse events, and comparable survival when compared with Cis-based and high-dose RT schemes.
We conducted a propensity-score weighted study to evaluate overall survival (OS) and safety of four contemporary CRT regimens in patients with proximal EC.
Material and methods
This multicenter, retrospective, observational study was conducted in 11 centers in The Netherlands. Patients were eligible if they were 18 years of age or older, diagnosed with squamous cell cancer of the proximal esophagus (i.e. <24 cm from incisor teeth [Citation21]), stage cT1N + M0 or cT2-4N0-3M0, and started with CRT treatment with a curative intent, between January 2004 and December 2014. Tumor staging was performed according to the TNM 6th or 7th edition based on date of diagnosis [Citation21,Citation22]. Patients with supraclavicular lymph node metastasis were included, as these lymph nodes were considered to be locoregional node metastasis [Citation23]. Patient informed consent was waived by the Medical Ethics Board azM/UM due to the retrospective nature of the study (METC 15-4-012). The study was approved by the scientific committee of the Dutch Upper GI Cancer Group (DUCG), and the Dutch Head and Neck Oncology Cooperative Group (NWHHT 2017-01). The data that support the findings of this study are available from the corresponding author upon request.
Treatment
Patients received a CRT regimen consisting of cisplatin (Cis)-based chemotherapy with low (Cis-low-dose RT) or high (Cis-high-dose RT) dose RT, or carboplatin-paclitaxel (CP)-based chemotherapy combined with low (CP-low-dose RT) or high (CP-high-dose RT) dose RT.
Cis-based chemotherapy included regimens with Cis 100 mg/m2 (day 1) administered at weeks 1, 4, and 7 of RT; Cis 6 mg/m2 daily during the first 25 fractions of RT; Cis 40 mg/m2 weekly during RT; or Cis 75 mg/m2 (day 1) plus 5-FU 1 g/m2 (days 1–4) at weeks 1 and 5 of RT with or without two additional courses on weeks 8 and 11. CP regimens comprise carboplatin AUC2 and paclitaxel 50 mg/m2 administered weekly during the period of RT.
RT groups were categorized into a low-dose group with a planned radiation dose above 41.4 Gy, as used in neoadjuvant schedules, and not exceeding 50.4 Gy, and a high-dose group with a RT dose above 50.4 Gy, in daily fractions of 1.8–2 Gy. Patients were treated by means of external-beam radiation, usually given with 4–6 MV photon linear accelerators. RT was given by a standard 3 or 4-field technique or by an intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) technique depending on availability in the treatment period. Depending on local institutional policies, the Gross Tumor Volume (GTV) of the primary tumor was delineated on the planning-CT and expanded to a Clinical Target Volume (CTV) by a margin of up to 3 cm in craniocaudal direction. Involved lymph nodes were also delineated in the CT scan and expanded to a CTV. The peri-esophageal area at the level of the CTV was included to ensure inclusion of adjacent draining lymph nodes. In cervical EC (CEC), sometimes Elective Nodal Irradiation (ENI) was added to include parts of the (lower) neck levels. The CTV’s were combined and expanded by a margin up to 1 cm into a Planning Target Volume (PTV). Radiation dose was prescribed to the PTV.
Data collection
All consecutive patients with proximal esopageal cancer and treated with definitive CRT were identified from the population-based Netherlands Cancer Registry. Next, we collected the data from the medical records of the Radiotherapy institutes and Medical Oncology and Surgical Oncology departments. Patient demographics, tumor characteristics, treatment details, tumor response, and vital status were collected retrospectively from the medical records. Data collection was performed between April 2017 and May 2018.
Outcomes
The primary endpoint was OS, defined as the period from start date of RT to the date of death from any cause, or censored at the date of last follow-up. Secondary endpoints included safety, and tumor response three months after completing CRT as assessed by the local study investigators by means of clinical investigation and/or imaging. Patients were generally examined in regular follow-up according to national guidelines at 4–8 weeks after completion of CRT, and every 3 months in the first year, with escalating interval up to 5 years or until death. Safety was scored by the study investigators using Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 [Citation24]. Acute toxicity was defined as adverse events occurring during CRT or within 90 days after last radiation dose. Late toxicity was scored if the toxic event appeared at least 90 days after the end of therapy.
Statistical analyses
Baseline characteristics were analyzed using the ANOVA test or Kruskal–Wallis test for continuous variables, and the Chi-squared test or Fisher exact test for categorical variables. Median follow-up was estimated using the inverse Kaplan–Meier method. OS was calculated by the Kaplan–Meier method. Differences in OS between the CRT regimens were determined using the logrank test, and univariably and multivariably in Cox proportional hazards regression models. OS analyses were performed based on an intention-to-treat analysis, i.e. all included patients, and a per-protocol analysis, including patients who completed treatment as planned, defined as undergoing all fractions of RT and all cycles of chemotherapy, excluding dose interruption or reductions.
For the multivariable analyses of OS, both a classical model as well as a post hoc propensity score (PS) weighted model were used, because the events per variable ratio was borderline for a classical model, i.e. eight (all variables considered) to twelve (multivariable model) [Citation25]. Confounding factors included in the PS weighted model were age, gender, WHO performance status, comorbidity, cT stage, cN stage, tumor location and tumor length. Variables with p < .10 in the univariable analysis were subsequently selected for the classical Cox regression analysis. Cis-low-dose RT was assigned as reference group, regarded as standard for definitive CRT regimen in EC [Citation8]. Considering the numbers of covariables, PS weighting was performed to balance the pretreatment covariable distributions of the different treatment groups. First, a PS for each patient was calculated by generalized boosted regression using the same variables as included in the univariable model. Four different stopping rules, based on summary statistics (i.e. maximum or mean) of absolute standardized bias or the Kolmogorov–Smirnov statistic which compare the means or the distributions of the covariates between treatment groups [Citation26,Citation27], overlap between the groups, and the balance was investigated. After calculating the PS weights, they were used in a weighted survival analyses to calculate the effect of the different treatments on OS. Because of the small sample size of the CP-high-dose RT group (N = 12), sensitivity analyses were performed excluding this group.
Differences in response and (grades 3–5) adverse events were evaluated using the Chi-square test. Additionally, a multilevel (patients within centers) logistic regression analysis on grades 3–5 acute and late toxicity by definitive CRT regimen was performed, including calender period and GTV (surrogate for RT field size) on patient level as confounding factors. Since causality of adverse events with either radiotherapy or chemotherapy could not be distinguished in most cases, no separate analysis of toxicity for treatment modality was performed.
Results
In total, 200 patients were included. Fifty-four patients (26%) underwent Cis-low-dose RT, 39 patients (19.5%) Cis-high-dose RT, 95 patients (47.5%) CP-low-dose RT, and 12 patients (6%) CP-high-dose RT. Cis-based therapy was replaced by CP in most sites after 2010 (Supplementary Figure S1). Baseline characteristics are presented in . Median age was higher in the CP-low-dose RT group compared with the other groups (p = .02). Comorbidity frequencies were comparable between treatment groups (p = .27). Patients in the Cis-high-dose RT group had the lowest probability of completing CRT (59%). In the CP-low-dose RT group, 93% of the patients received 50.4 Gy, and 98% in the Cis-low dose RT group received 50–50.4 Gy (data not shown). Six of the 67 patients with an incomplete response underwent salvage surgery.
Table 1. Baseline characteristics of 200 consecutive patients with proximal esophageal cancer treated with definitive chemoradiation (2004–2014).
Survival
Median follow-up of all patients was 62.6 months (95% confidence interval [CI]: 47.9–77.2 months). During the study period, 139 deaths occured including 96 (69%) due to sequelae of the esophageal tumor, 6 (4%) as a result of toxicity, 18 (13%) due to other causes, and 19 (14%) due to unknown causes. Median OS was 21.9 months (95% CI: 16.9–27.0 months) for the total population, and 21.9 (95% CI: 17.7–26.1 months), 17.2 (95% CI: 0.0–48.1 months), 23.2 (95% CI: 15.6–30.8 months), and 15.7 (95% CI: 8.3–23.2 months) months in Cis-low-dose RT, Cis-high-dose RT, CP-low-dose RT, and CP-high-dose RT groups, respectively (, p logrank = .88). Three-year OS rates were 35% (95% CI: 22–48%) in the Cis-low-dose RT group compared to 46% (95% CI: 30–61%), 40% (95% CI: 30–50%), and 33% (95% CI: 10–59%), in Cis-high-dose RT, CP-low-dose RT, and CP-high-dose RT group, respectively. Per-protocol analysis showed comparable results (Supplementary Figure S2, p logrank = .76).
Figure 1. Kaplan–Meier curves for overall survival by treatment, intention-to-treat analysis. Cis/low: cisplatin and low-dose radiotherapy; Cis/high: cisplatin and high-dose radiotherapy; CP/low: carboplatin/paclitaxel and low-dose radiotherapy; CP/high: carboplatin/paclitaxel and high-dose radiotherapy.
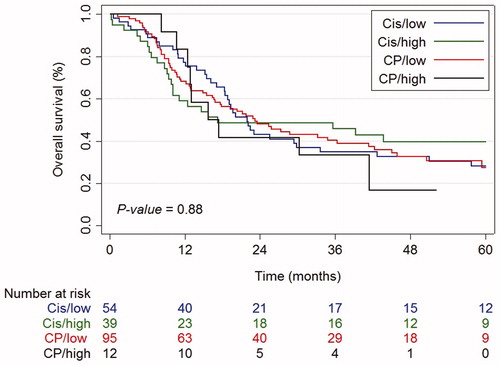
After correction for potential confounders, no difference in OS between the four treatment schemes was observed (). Male gender, WHO performance status 2-3, unknown T stage (Tx), and N + stage were independent unfavorable prognostic factors for OS. OS was significantly higher in patients with tumors located in the upper thoracic segment compared to patients with cervical tumors [hazard ratio (HR) 0.61, 95% CI: 0.42–0.88]. The PS weighted cox regression analysis showed no significant difference in OS between treatment groups. Sensitivity analyses excluding CP-high-dose RT group showed a better balance of the PSs and overlap between the groups, confirming that there were no statistically significant differences in OS.
Table 2. Univariable and multivariable comparison of overall survival by four definitive chemoradiation regimens, and propensity score adjusted for overall survival.
Univariable analysis showed no difference in OS between patients treated with Cis-based versus CP-based regimens (p = .81), or between low-dose versus high-dose RT (p = .64) (data not shown). Both patients with CEC or upper thoracic EC (UTEC) showed comparable OS between treatment groups (p = .90 and p = .92, respectively) (Supplementary Figure S3).
Tumor response
Response to therapy was available in 196 patients (98%) (Supplementary Table S1). Clinical complete response was achieved in 57%, 69%, 68%, and 75% of patients in the Cis-low-dose RT, Cis-high-dose RT, CP-low-dose RT, and CP-high-dose RT group, respectively. The rate of complete response versus incomplete response was not significantly different between the groups (p = .72). The rate of partial response was comparable for the different regimens, varying between 18 and 25%.
Adverse events
Grades 3 or 4 acute adverse events occurred in 22 (41%), 19 (49%), 21 (22%), and 5 (42%) patients in Cis-low-dose RT, Cis-high-dose RT, CP-low-dose RT, and CP-high-dose RT group, respectively (). Dysphagia was the main contributor of grades 3 or 4 toxicity in all groups. The incidence of acute renal failure was higher in the Cis-groups compared with the CP-groups. Comparable rates of treatment-related deaths occurred in all four groups. In total, six patients died because of treatment-related adverse events, of which two patients during CRT and the remaining with an interval of 4.4–10.4 months after start of CRT. Three patients died from respiratory problems, one from esophageal stenosis, one from esophageal perforation, and one from esophageal fistulation. The incidence of grades 3–5 acute adverse events was significantly lower in patients treated with CP-low-dose RT compared to patients treated with one of other three regimens (p = .01). The multivariable multilevel analysis confirmed a significantly better safety profile in terms of acute toxicity of CP-low-dose RT versus Cis-high-dose RT (OR 3.78 (95% CI: 1.31–10.87); p = .01), and a trend toward a favorable safety profile of CP-low-dose RT versus Cis-low-dose RT (p = .10, ). The occurrence of grades 3–5 late toxicities was not different between treatment groups (p = .32), confirmed in multivariable analysis ().
Table 3. Adverse events, No. (%).
Table 4. Multivariable multilevel (patients within centers) analyses of grades 3–5 acute and late toxicity.
Discussion
Definitive CRT is the current standard of care in patients with proximal EC [Citation7,Citation8]. Although there seems to be general consensus regarding the need for CRT, there is significant variation in the design of this multimodality therapy. In this study, no statistically significant difference in OS could be determined between four contemporary CRT regimens. A firm conclusion is however not possible due to the small sample size. However, CP showed a better safety profile compared with Cis-based groups.
Limited randomized series on CRT regimens in EC investigated either unknown numbers of proximal EC patients [Citation28], or limited numbers of patients with proximal EC [Citation29,Citation30]. A recent phase 3 trial in patients with EC treated with definitive CRT showed no significant difference in OS between paclitaxel plus 5-FU and Cis plus 5-FU [Citation14]. This led to the initiation of a phase 3 trial comparing paclitaxel plus cisplatin, CP, and paclitaxel plus 5-FU concurrent with RT for patients with EC (NCT02459457). No randomized trials have been published comparing Cis- versus CP-based CRT schemes in patients with EC.
RT dose escalation (64.8 versus 50.4 Gy) was evaluated in patients with EC in a randomized trial (INT-0123/RTOG 94-05) [Citation28]. This trial was prematurely stopped when an interim analysis showed a higher treatment-related mortality rate in the high-dose RT arm. Yet, seven of the 11 deaths occurred in patients who actually had received ≤50.4 Gy. A recent meta-analysis (n = 3736) including the before mentioned RTOG 94-05 trial and seven retrospective studies demonstrated better outcomes in patients with squamous cell EC treated with definitive CRT with high dose radiotherapy (≥60 Gy) versus 45–59.4 Gy [Citation31]. However, this analysis excluded patients with incomplete dosage, receiving <45 Gy. In the era of modern RT techniques, dose escalation in CRT for EC has been investigated in 260 patients with EC by the randomized ARTDECO study, including 72 patients (28%) with proximal EC. Radiation dose escalation up to 61.6 Gy versus 50.4 Gy to the primary tumor in CP-based CRT did not improve local control and OS [Citation32].
To the best of our knowledge, this is the largest study comparing Cis- versus CP-based CRT, with RT dose-escalation regimens in patients with proximal EC in a curative treatment approach. OS data in our cohort is consistent with results of previous observational studies in cervical or proximal EC exploring the effects of CRT, with 3-year OS rates of about 35–45% [Citation33–39]. Recently, a small retrospective single institutional Korean study exploring the impact of radiation dose escalation on OS showed more favorable 3-year OS rates compared to the results in the current study, with 58% in the high-dose RT (≥59.4 Gy) and 49% of patients in the low-dose RT group (<59.4 Gy, p = .69) [Citation40]. However, this study excluded patients who had not completed treatment as planned. In line with our results, an analysis of 789 patients with CEC from the US Cancer Data Base demonstrated no association between radiation dose escalation and improved OS [Citation41]. Of note, they did not provide information on potential confounding factors, such as chemotherapeutic regimens and safety information. Furthermore, one Japanese phase 2 trial [Citation42] was conducted in 2009–2013 in 30 patients with CEC to evaluate Cis-based high dose RT (60 Gy), demonstrating a remarkable 3-year OS of 67%, compared with 46% observed in this study. However, this concerned a highly selected patient group with mostly early stage disease and excellent performance status. Interestingly, in this series one-third of the patients underwent salvage surgery. With a comparable complete response rate of 73%, the higher 3-year OS outcome suggests a possible role for salvage surgery in patients who fail to achieve a complete response.
Our study confirmed previous data concerning impaired treatment compliance in patients treated with Cis-based CRT. Honing et al. [Citation20] described that only 57% of patients with EC treated with Cis-based CRT completed treatment, comparable with 59% of patients treated with Cis-high-dose RT in our cohort. Remarkably, 82% of the patients in the current study undergoing Cis-low-dose RT were able to complete therapy. To help determine if non-adherence to Cis-high-dose RT was responsible for OS in this group, a separate per-protocol analysis was performed that included only patients who completed the assigned CRT schedule (Supplementary Figure S2). Despite this biased analysis, again no survival advantage of the Cis-high-dose RT group could be demonstrated. Premature treatment discontinuation was frequently a result of side effects. Considering this study was unable to provide survival differences between the four treatment groups, arguments for treatment choice might be based on the expected adverse event rates.
The safety profile of CP-low-dose RT group was more favorable than that of the Cis-based groups. One in five patients treated with CP-low-dose RT experienced grades 3 or 4 acute adverse events, whereas half of the patients treated in the other three groups did. In agreement, retrospective studies in primarily thoracic EC demonstrated that CP-based CRT was better tolerated compared with Cis-based CRT, again with comparable survival [Citation20,Citation43]. The reported toxicity rates in the CP-based CRT groups in our study demonstrated higher treatment-related grade 3 or greater toxic event rates, as compared with data from the CP-based CRT group in the randomized neoadjuvant CROSS trial [Citation15]. Accordingly, we consider these toxicity data to be registered accurately. In addition, multilevel logistic regression showed that higher toxicity rates were registered in patients treated in the more recent years, independent of CRT regimen (). Hence, since CP-based regimens were more common in the contemporary era, and underreporting is expected to be higher in the older years (OR 5.64 (95% CI: 1.66–19.16) of grades 3–5 acute toxicity in 2010–2014 versus 2004–2006), the observed difference in toxicity between Cis- and CP-based regimens may in fact be larger than retrospectively observed in this study.
In addition to the more favorable toxicity profile, CP does not require inpatient protective hydration, in contrast to high-dose Cis [Citation44]. Moreover, health-related costs can be reduced by a shift to outpatient treatment, and as such CP-based regimens may be preferred for everyday clinical practice.
The strength of this study is that we included a relatively large number of patients with a rare disease. Since a randomized controlled trial in a disease with this low incidence is challenging, we provide the second best study design. Moreover, this study collected long-term follow-up data.
The retrospective design of this study is inherent with some limitations. The observational nature of the study makes it sensitive for bias, such as selection bias. However, correction for institution in multilevel logistic regression analysis did not alter the odds of the occurrence of grade 3-5 toxicities per treatment regimen. We were able to realize a fair sample size by including 200 patients with proximal EC. According to the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS), a HR ≤ 0.70 is defined as a clinically meaningful benefit for treatments that are not likely to be curative [Citation45]. Although in our study, the point estimates of the HR for the different regimens approximates one (suggesting a possible OS difference would be small), the CI’s include 0.70, meaning that our study is not powered to state that there is no difference. We invite other institutions and countries to study this question as well, in order to perform a meta-analysis in the future.
In conclusion, the small sample size of this study restricts a definitive conclusion regarding OS differences between the CRT regimens. Based on the superior safety profile, in addition to a more feasible outpatient implementation, we suggest a CRT regimen with carboplatin and paclitaxel in the curative setting for patients with proximal EC.
Supplemental Material
Download PDF (116.9 KB)Acknowledgments
In addition to the authors, we would like to thank the following co-workers of this trial; S Eerenstein, J Heijnen-Mommers, E J M de Jong, M Kloft, L Nijssen, M Noordhoek, M van der Sangen, H Spruijt, L Valkenburg-van Iersel, F Warmerdam. We had no writing assistance in writing this manuscript.
Disclosure statement
JV-G has received non-financial support from BTG, and Servier, and has received institutional research funding from Servier, all outside the submitted work.
CTM has received institutional research funding from IBA RaySearch, Siemens, Elekta and Mirada, all outside the submitted work.
HWML has served as a consultant for BMS, Celgene, Lilly, Nordic, and Servier and has received unrestricted research funding from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips, Roche, and Servier, all outside the submitted work.
VEPPL has received an unrestricted research grant and educational grant from Roche, outside the submitted work.
VCGT-H has received honoraria/travel grants from Roche, Novartis, Pfizer, Lilly, and Accord Healthcare, and has received institutional research funding from AstraZeneca, Roche, Pfizer, Novartis, Eisai, and Lilly, all outside the submitted work.
GAPN has received honoraria/travel grants from Medtronic, outside the submitted work.
All remaining authors have declared no conflicts of interest.
References
- van Putten M, de Vos-Geelen J, Nieuwenhuijzen GAP, et al. Long-term survival improvement in oesophageal cancer in the Netherlands. Eur J Cancer. 2018;94:138–147.
- Grass GD, Cooper SL, Armeson K, et al. Cervical esophageal cancer: a population-based study. Head Neck. 2015;37(6):808–814.
- Valmasoni M, Pierobon ES, Zanchettin G, et al. Cervical esophageal cancer treatment strategies: a cohort study appraising the debated role of surgery. Ann Surg Oncol. 2018;25(9):2747–2755.
- Takebayashi K, Tsubosa Y, Matsuda S, et al. Comparison of curative surgery and definitive chemoradiotherapy as initial treatment for patients with cervical esophageal cancer. Dis Esophagus. 2017:30(2):1–5.
- Cao CN, Luo JW, Gao L, et al. Primary radiotherapy compared with primary surgery in cervical esophageal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(10):918–926.
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623–1627.
- National Comprehensive Cancer Network. NCCN Guidelines®. NCCN Guidelines® & Clinical Resources 2018. Esophageal and Esophagogastric Junction Cancers (Version 2.2018) 2018 [updated May 22, 2018]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v50–v57.
- Hoeben A, Polak J, Van De Voorde L, et al. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol. 2016;27(9):1664–1674.
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169–175.
- Pignon JP, Le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14.
- Strojan P, Vermorken JB, Beitler JJ, et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: a systematic review. Head Neck. 2016;38(S1):E2151–E2158.
- Herskovic A, Martz K, Al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–1598.
- Chen Y, Ye J, Zhu Z, et al. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: a randomized, multicenter, phase III clinical trial. JCO. 2019;37(20):1695–1703.
- van Hagen P, Hulshof M, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084.
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997 ;337(3):161–167.
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. JCO. 2008;26(7):1086–1092.
- Lee JL, Park SI, Kim SB, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15(6):947–954.
- Jeene PM, van Laarhoven HWM, Hulshof M. The role of definitive chemoradiation in patients with non-metastatic oesophageal cancer. Best Practice Res Clin Gastroenterol. 2018;36-37:53–59.
- Honing J, Smit JK, Muijs CT, et al. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol. 2014;25(3):638–643.
- Greene FL, Balch Cm Haller Dg, et al. AJCC Cancer Staging Manual, 6th edn. New York: Springer-Verlag; 2002.
- Edge SB. Byrd Dr, Compton CC. The AJCC Cancer Staging Manual. 7th edn. New York: Springer. 2010.
- Jeene PM, Versteijne E, van Berge Henegouwen MI, et al. Supraclavicular node disease is not an independent prognostic factor for survival of esophageal cancer patients treated with definitive chemoradiation. Acta Oncologica (Stockholm, Sweden. 2017;56(1):33–38.
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), v4.03; 2009 [cited 2010 Jun 14]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- Biondi-Zoccai G, Romagnoli E, Agostoni P, et al. Are propensity scores really superior to standard multivariable analysis?. Contemp Clin Trials. 2011;32(5):731–740.
- Burgette L, Griffin BA, McCaffrey D. Propensity scores for multiple treatments: a tutorial for the mnps function in the twang package. 2020. Available from: https://cran.r-project.org/web/packages/twang/vignettes/mnps.pdf
- McCaffrey DF, Griffin BA, Almirall D, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Statist Med. 2013;32(19):3388–3414.
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167–1174.
- Conroy T, Galais M-P, Raoul J-L, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. The Lancet Oncol. 2014;15(3):305–314.
- Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. The Lancet Oncol. 2013;14(7):627–637.
- Luo HS, Huang HC, Lin LX. Effect of modern high-dose versus standard-dose radiation in definitive concurrent chemo-radiotherapy on outcome of esophageal squamous cell cancer: a meta-analysis. Radiat Oncol. 2019;14(1):178.
- Hulshof M, Geijsen D, Rozema T, et al. A randomized controlled phase III multicenter study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer: ARTDECO study. JCO. 2020;38(4_suppl):281–281. Abstract 281.
- Huang SH, Lockwood G, Brierley J, et al. Effect of concurrent high-dose cisplatin chemotherapy and conformal radiotherapy on cervical esophageal cancer survival. Int J Radiat Oncol Biol Phys. 2008;71(3):735–740.
- Zhang P, Xi M, Zhao L, et al. Clinical efficacy and failure pattern in patients with cervical esophageal cancer treated with definitive chemoradiotherapy. Radiother Oncol. 2015;116(2):257–261.
- Herrmann E, Mertineit N, De Bari B, et al. Outcome of proximal esophageal cancer after definitive combined chemo-radiation: a Swiss multicenter retrospective study. Radiat Oncol. 2017;12(1):97.
- Gkika E, Gauler T, Eberhardt W, et al. Long-term results of definitive radiochemotherapy in locally advanced cancers of the cervical esophagus. Dis Esophagus. 2014;27(7):678–684.
- Yamada K, Murakami M, Okamoto Y, et al. Treatment results of radiotherapy for carcinoma of the cervical esophagus. Acta Oncologica (Stockholm, Sweden). 2006;45(8):1120–1125.
- Uno T, Isobe K, Kawakami H, et al. Concurrent chemoradiation for patients with squamous cell carcinoma of the cervical esophagus. Dis Esophagus. 2007;20(1):12–18.
- Ludmir EB, Palta M, Zhang X, et al. Incidence and prognostic impact of high-risk HPV tumor infection in cervical esophageal carcinoma. J Gastrointest Oncol. 2014;5(6):401–407.
- Kim TH, Lee IJ, Kim Jh, et al. High-dose versus standard-dose radiation therapy for cervical esophageal cancer: retrospective single-institution study. Head Neck. 2019;41(1):146–153.
- De B, Rhome R, Doucette J, et al. Dose escalation of definitive radiation is not associated with improved survival for cervical esophageal cancer: a National Cancer Data Base (NCDB) analysis. Dis Esophagus. 2017;30(4):1–10.
- Zenda S, Kojima T, Kato K, et al. Multicenter phase 2 study of cisplatin and 5-fluorouracil with concurrent radiation therapy as an organ preservation approach in patients with squamous cell carcinoma of the cervical esophagus. Int J Rad Oncol Biol Phys. 2016;96(5):976–984.
- Blom RL, Sosef MN, Nap M, et al. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Dis Esophagus. 2014;27(4):380–387.
- Ozols RF, Corden BJ, Jacob J, et al. High-dose cisplatin in hypertonic saline. Ann Intern Med. 1984;100(1):19–24.
- Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2017;28(11):2901–2905.
