Abstract
Objectives: The aims of this study were to compare patients 70 years or older with younger patients, to examine whether Danish patients with early-stage breast cancer aged 70 years or more received treatment according to guidelines, the reasons for deviating from the guidelines, and to analyze whether such deviations affected survival.
Methods: From the Danish Breast Cancer Cooperative Group (DBCG) database we identified 23,247 women diagnosed with early-stage breast cancer in Denmark from 2008 to 2012. 17,391 were aged less than 70 years and 5856 were 70+ years. We reviewed medical charts of 441 patients aged 70+ years from Funen (a region of Denmark) to ascertain whether treatment was given according to the guidelines of DBCG and if not, the reason for deviating. Overall survival was analyzed by Cox proportional hazards models.
Results: Up to age 80 years most women (94%) had surgery according to guidelines, decreasing to 41% in women aged 85+ years, the main reason for omitting surgery being patients’ requests. Patients with breast cancer over the age of 80 years did not have an excess mortality compared with the general population in Funen. Compared with women who had surgery according to guidelines, women who did not have surgery had a significantly higher risk of dying with a hazard ratio (HR) of 8.38 (95% Confidence Intervals (CI) 4.46–15.8) if they were less than 80 years and HR = 2.56 (95% CI 1.63–4.01) if they were 80 years or more (p = .003 for interaction).
Conclusions: Adherence to treatment according to guidelines decreases with increasing age, mainly for patients aged 80+ years. Our results suggest that surgery is important for the survival of patients aged less than 80 years.
Introduction
Although less than a third of all breast cancers are diagnosed after the age of 70 years, breast cancer remains the most frequent malignant disease among women regardless of age [Citation1,Citation2]. The prognosis after breast cancer is determined by patient characteristics, such as age and comorbidities; disease related factors, such as stage of disease; and treatment. Recent data from Denmark demonstrate that the relative survival decreases with age above 70 years [Citation3]. The proportion of patients with comorbidities increases with age from 13% of patients aged 50–59 years to 40% among patients aged 80 years or more [Citation4]. Both age and presence of comorbidities may affect stage of disease and choice of treatment. Staging procedures like axillary dissection are performed less frequently in older patients with comorbidities and more of these patients are classified as having an unknown stage of disease [Citation5], but with proper staging, tumor characteristics do not appear to differ across categories of Charlson Comorbidity Index [Citation4,Citation6].
Guidelines for adjuvant breast cancer treatment tend to be based on evidence from randomized clinical trials. However, these trials include mainly patients younger than 70 years and free from comorbidity. The meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) included a total of 44,251 women in trials of taxanes versus non-taxane based adjuvant chemotherapy of whom only 657 or 1.5% were 70 years or older but the reduction in risk of death was similar or greater than among younger women [Citation7]. Up to 2007, the Danish Breast Cancer Cooperative Group (DBCG) did not recommend chemotherapy for patients aged more than 70 years. Several studies have shown that older patients, particularly those over 80 years, were less likely to receive treatment according to the guidelines [Citation5]. However, a recent Dutch study failed to show an association between overall survival and adherence to treatment guidelines [Citation8].
The aims of this study were to compare patient and disease characteristics in patients 70 years or older with younger patients, to examine the extent to which Danish patients with early-stage breast cancer over the age of 70 years received treatment according to the Danish guidelines, the reasons for deviating from the guidelines, and to analyze whether such deviations affected survival.
Method
Study design and data collection
Since 1977, the DBCG has performed clinical trials and issued national guidelines for adjuvant breast cancer treatment. Data have been collected on diagnosis, histopathology, and treatment for all newly diagnosed early-stage breast cancer patients in Denmark. Early stage was defined as potentially operable, non-metastatic breast cancer. The DBCG database is considered as a valid database with 95% completeness [Citation9]. Estrogen receptor (ER) status and expression of Human Epidermal Growth Factor Receptor 2 (HER-2) was measured by immunohistochemistry and in situ hybridization according to national guidelines at the time of diagnosis.
From the database of the DBCG we identified a total of 23,247 women diagnosed with early-stage breast cancer in Denmark between 2008 and 2012, of whom 17,391 were aged less than 70 years and 5856 were 70 years or more. In the latter group, 463 patients were treated in the island of Funen, covering about 9% of the Danish population. We excluded eighteen patients because of metastatic disease at diagnosis, two patients with recurrence of earlier breast cancer, and two patients due to missing data ().
Figure 1. 23,247 women from the Danish Breast Cancer Cooperative Group (DBCG) database diagnosed with early-stage breast cancer in Denmark, 2008–2012 according to age at diagnosis and residence.
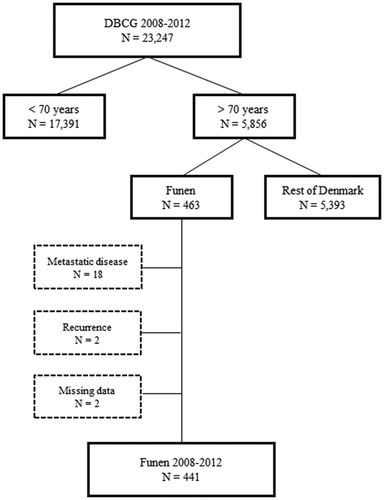
Among 441 women treated in Funen, we ascertained whether surgery, radiotherapy, chemotherapy, endocrine therapy, and trastuzumab were indicated according to the Danish guidelines for early stage breast cancer at that time [Citation10]. Guideline surgery was defined as either a radical mastectomy or breast conserving surgery, both with proper axillary surgery, i.e. sentinel node biopsy or axillary lymph node dissection if metastasis were detected. Other guideline treatment was defined as the patient initiating the treatment regimen as indicated in the guidelines. After approval from the Danish Patient Safety Authority we abstracted all medical charts for information on whether the indicated treatment was actually given. If guideline therapy was not given, the reason for deviating from the guidelines was registered. After assessment of the first fourth of the medical charts it became evident that reasons for deviating from guideline therapy could be divided into three main reasons: age, patient request, or comorbidity. Age meant that the doctor thought that the patient was too old to benefit from the treatment. Patient request was used if the patient did not wish the treatment or considered herself being too old to go through the treatment. Comorbidity indicated that the patient suffered from other diseases than breast cancer which made the doctor reluctant to prescribe the treatment indicated. In cases of more than one reason for deviating from guideline treatment we registered the reason which seemed of greatest importance in the specific case.
Statistical analysis
Patient and treatment characteristics were compared according to study area (Funen vs rest of Denmark) and age group (<70 vs ≥70) using the χ2-test excluding unknowns. Overall survival (OS) was defined as the interval from surgery/biopsy until death, with all causes of death considered an event. Follow-up for death irrespective of clinical follow-up was achieved until June 1, 2017 by linkage to the Danish Central Population Registry. Kaplan Meier estimates were calculated for overall survival. The Cox proportional hazards regression model was applied to quantify the prognostic effect of age and treatment modalities for the end-point of overall survival. No further parameters were considered due to sample size. Interaction between age and each of the treatment modalities were investigated in separate models by applying the Wald test. Proportional hazards assumptions were assessed using Schoenfeld residuals. The expected number of deaths was estimated by multiplying the survival time accrued from the study cohort by the mortality rates obtained from Statistics Denmark of the general population of women matched by age (1-year groups) and calendar period (1-year groups). Statistical analyses were done using the SAS 9.4 software program package (SAS Institute, Cary, NC) and Stata/IC 14.2 (StataCorp).
Results
Patient and tumor characteristics were compared in two groups, those who had a biopsy only (N = 1204) and those who had surgery (N = 22,043) (). A biopsy only without subsequent surgery was performed in less than 2% of patients aged less than 70 years but in 15% of patients aged 70 years or more. ER-positive patients in this group were treated with primary endocrine therapy. Differences in tumor characteristics were observed between younger and older patients in the biopsy only group, most of them due to a higher proportion of unknowns among those aged 70 years or more ().
Table 1. Characteristics of 23,247 women diagnosed with early-stage breast cancer in Denmark 2008–2012, by age and residence at diagnosis.
In the surgery group (N = 22,043), significantly fewer older women had breast conserving surgery compared with younger (52% vs. 70%, p = <.0001). Older women had less axillary surgery (axillary dissection and sentinel node biopsy) than the younger and more often no axillary surgery (11% vs 4.9%, p = <.0001). In addition, surgery was less radical regarding the margins in older compared with younger, tumor size was larger, histology more often lobular, and malignancy grade higher, all differences being statistically significant. ER status was quite similar in older and younger women whereas fewer older women had overexpression of HER-2 compared to the younger women. Overall, women aged 70 years or more in Funen were comparable with women aged 70 years or more in the rest of Denmark apart from having significantly more mastectomies (60% vs 48%, p = <.0001).
shows adherence to guideline treatment and reasons for deviation among 441 women according to age from Funen. Most women aged less than 80 years (94%) had surgery according to guidelines but the proportion decreased with increasing age to 41% among women aged 85 years or more, the main reason for omitting surgery being patients’ request. Among patients for whom radiation treatment was indicated, a similar trend was observed with decreasing adherence with increasing age, the proportions completing radiation treatment if indicated being 82%, 65%, 42%, and 22% in the age groups 70–74, 75–79, 80–84, and 85+ years respectively, with an even distribution of reasons for omitting radiation treatment. Most patients (94%) aged less than 80 years received endocrine therapy if indicated, decreasing to 77% in women aged 85 years or more. Old age and comorbidity were given as reasons for not offering endocrine therapy. Chemotherapy was not indicated for 78–91% of the older patients and no patient over the age of 80 years received adjuvant chemotherapy. Similar figures were seen for trastuzumab.
Table 2. Guideline treatment and reasons for deviations among 441 women according to age at diagnosis of early-stage breast cancer in Funen 2008–2012.
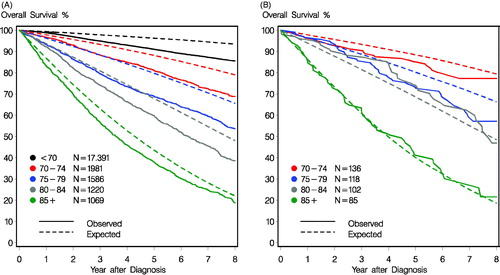
illustrates that the survival observed among women diagnosed with early-stage breast cancer in Denmark between 2008–2012 (N = 23,247) was lower than the expected for the general female population, but that the relative difference between the observed and expected survival diminished with increasing age. Among women treated in Funen (), the survival observed in among 102 breast cancer patients aged 80–84 years was actually better than the expected while there was no difference between the observed and expected survival among 85 women aged 85 years or more.
Figure 2. (A) The observed and expected survival for all women diagnosed with early stage breast cancer between 2008-2012 according to age at diagnosis (N = 23,247). (B) The expected and observed survival among the older women diagnosed with early stage breast cancer in Funen between 2008–2012 according to age at diagnosis (N = 441). 5 year estimated overall survival (95% confidence intervals) was: 85% (78–92), 74% (65–81), 75% (66–83), and 41% (31–52) for the age groups: 70–74 years, 75–79 years, 80–84 years, and 85+ years, respectively.
Using Cox regression models, we examined the prognostic effect of age and treatment modalities on overall survival in women treated in Funen (). Except for surgery, we detected no significant interactions between age and treatment modalities. Compared with women who had surgery according to guidelines, women who did not have surgery had a significantly higher risk of dying with adjusted estimates of hazard ratio (HR) of 8.38 (95% Confidence Intervals (CI) 4.46–15.8) if they were less than 80 years and HR = 2.56 (95% CI 1.63–4.01) if they were 80 years or more (p = .003 for interaction).
Table 3. Overall survival after early stage breast cancer among women aged 70 years or more in Funen between 2008–2012, adjusted for age.
Discussion
Overall adherence to treatment guidelines decreased with increasing age among older women with early-stage breast cancer in Denmark. The main reason for deviating from treatment guidelines was the patients’ request. Compared with women who had surgery according to guidelines, women who did not have surgery had a significantly higher risk of dying with a hazard ratio (HR) of 8.38 if they were less than 80 years and HR = 2.56 if they were 80 years or more.
Based on data from the entire Danish population, our findings agree well with those of other studies [Citation11–15] showing that breast cancer patients aged 70 years or more are diagnosed with more advanced disease (larger tumors, more often node positive) than younger patients. Our results also confirm that older patients receive different surgery with more biopsies only, more mastectomies than breast conserving surgery, and less axillary surgery. Across Europe, there is a substantial variation in treatment of patients aged 70 years or more with non-metastatic breast cancer [Citation14]. The proportion of patients receiving no breast surgery varied by country and stage of disease with the highest proportions seen for Stage I and II in England (24–28%) and Stage III in Ireland (51%). In our material 15% (889/5.856) did not have surgery which is considerably lower than any of the other European countries. Thus, our results are more in line with current recommendations from the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) [Citation16] stating that women aged more than 70 years should be offered the same surgery as younger women with standard of care being breast conserving surgery (BCS) plus radiotherapy or mastectomy with or without radiotherapy.
Our subgroup analysis of patients treated in Funen revealed that surgery was not performed according to guidelines in up to 6% of patients aged less than 80 years, increasing to almost 60% of patients aged 85 years or more. For more than 50%, patients´ request was the reason for deviating from treatment guidelines with respect to surgery. In a study designed similarly to ours with review of medical charts, Hamaker et al. [Citation17] reported that patients` choice was the reason for omitting surgery in 32% of the patients, but they were not able to retrieve the reason for omitting surgery in 68% of the patients. The high number of patients stating their own request as reason for omitting treatment could reflect a shared decision between physician and patient [Citation17].
The adherence to guidelines for postoperative radiotherapy was difficult to evaluate because the DBCG guidelines did not include patients aged more than 70 years before 2008. In 2008, the age criterion was modified so that the decision on postoperative radiotherapy in patients aged 75 years or more should be based on an individual evaluation, with a high focus on patient shared decision making [Citation10]. For the analysis, we made the assumption that guidelines for patients aged 70 years or more were the same as for those aged less than 70 years. With this reservation, our results were consistent with data from the US Surveillance, Epidemiology and End Results-Medicare dataset showing that older patients were less likely to receive guideline-concordant post-mastectomy radiotherapy for Stage III breast cancer [Citation18]. The most common reason for not offering postoperative radiotherapy was that the patient was considered too old to benefit from the treatment.
Although adherence to adjuvant endocrine therapy decreased with increasing age, most patients received endocrine therapy if indicated by the guidelines. Adjuvant chemotherapy was not indicated for the majority (83%) of the patients and no patient over the age of 80 years received adjuvant chemotherapy. Our observations are similar to those of a Dutch study [Citation19]. Fewer patients aged 70 years or more had HER-2-positive tumor than younger patients and adjuvant trastuzumab was also not given to patients aged 80 years or more.
Survival
Many studies reporting survival after cancer use the measure ‘relative survival’ where the observed survival is divided by the expected survival from the same population, matched by age, gender and calendar year. Relative survival can be interpreted as the survival if the cancer was the only cause of death [Citation1]. Here we chose to illustrate both the observed and the expected survival to examine the differences by age. It was not surprising that the observed survival decreased with increasing age, but we noted that the difference between the observed and expected survival diminished with increasing age with the smallest difference seen in women aged 85 years or more i.e. breast cancer among the oldest women was not associated with the same excess mortality as in younger women. In our subgroup from Funen, women aged 80–84 years had a higher survival than expected while no difference was seen for women aged 85 years or more. We excluded women with metastatic disease or missing data in the Funen cohort, but not in the full cohort, which may have affected the results. While the population of Funen is fairly representative of the general Danish population with respect to education, employment, and other social indicators [Citation20], it differs in one important aspect: mammography screening. This was introduced in Funen in 1993 but nationally not until 2008 [Citation21]. Since mammography screening reduces mortality, older women in Funen who have a screening history can be expected to have a lower mortality than other women in Denmark. Though the survival data were based on a small number of patients, they still represent a group of older patients with breast cancer who have no or a very small excess mortality from breast cancer.
Many guidelines, e.g. the St. Gallen International Expert Consensus Conference [Citation22], use such methods to define breast cancer patients who are at a low risk of recurrence. Based on Danish data [Citation23], the DBCG guidelines do not recommend adjuvant systemic treatment to women who do not have increased risk of mortality compared with age-matched women from the general population. As a consequence, non-adherence to guidelines is likely to be associated with a poorer survival confirmed in many studies [Citation12,Citation13,Citation15]. In our multivariate analysis, age and surgery were the main determinants of survival with a significant 8-fold higher mortality among women aged less than 80 years not receiving surgery according to guidelines compared with women who had surgery. However, this group constituted only 6% and may have been different from the majority of patients for reasons that we were not able to control for in the analysis. The excess in mortality for women < 80 years may not be due to no-surgery alone. Comorbidity as reason for omitting surgery must be taken into account as well. Among women >80 years the reason for omitting surgery was due to patient request, and the group may therefore not be effected by comorbidity to the same extent.
The strength of this study is that it was based on the entire Danish population. Our analysis of adherence to guidelines covered a geographically defined area, Funen, served by only one University hospital. However, there are limitations to single institution studies. The surgery was performed by a limited number of surgeons whose approach to older women with breast cancer may not be representative for the attitudes of surgeons from the entire country. The main limitation is that we cannot rule out entirely that there may have been some degree of confounding since the reason for particularly omitting surgery may be related to survival.
In conclusion, adherence to treatment according to guidelines decreases with increasing age, mainly for patients aged 80 years or more. The main reason for deviating from guidelines was the patients´ request. Our results suggest that surgery is important for survival for patients less than 80 years.
Author contributions
Concept and design: Marianne Ewertz, Marianne Vogsen. Data collection: Marianne Vogsen. Analysis and interpretation of data: Marianne Vogsen, Maj-Britt Jensen, Marianne Ewertz. Manuscript writing: Marianne Vogsen, Maj-Britt Jensen, Marianne Ewertz. Critical review and approval of final article: Marianne Vogsen, Camilla Bille, Anne Marie Bak Jylling, Maj-Britt Jensen, Marianne Ewertz.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Ewertz M, On behalf of the Academy of Geriatric Cancer Research (AgeCare), Christensen K, Engholm G, et al. Trends in cancer in the elderly population in Denmark, 1980-2012. Acta Oncologica (Stockholm, Sweden. 2016;55(sup1):1–6.
- GLOBOCAN. [cited 2020 Apr 20]. Available from: http://globocaniarcfr/. 2012.
- Jensen JD, On behalf of the Academy of Geriatric Cancer Research (AgeCare), Cold S, Nielsen MH, et al. Trends in breast cancer in the elderly in Denmark, 1980–2012. Acta Oncologica (Stockholm, Sweden). 2016;55(sup1):59–64.
- Land LH, Dalton SO, Jensen MB, et al. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res Treat. 2012;131(3):1013–1020.
- Land LH, Dalton SO, Jorgensen TL, et al. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol/Hematol. 2012;81(2):196–205.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet (London, England). 2012;379(9814):432–444.
- van de Water W, Bastiaannet E, Dekkers OM, et al. Adherence to treatment guidelines and survival in patients with early-stage breast cancer by age at diagnosis. Br J Surg. 2012;99(6):813–820.
- Christiansen P, Ejlertsen B, Jensen MB, et al. Danish Breast Cancer Cooperative Group. CLEP. 2016;8:445–449.
- Jensen MB, Laenkholm AV, Offersen BV, et al. The clinical database and implementation of treatment guidelines by the Danish Breast Cancer Cooperative Group in 2007-2016. Acta Oncologica (Stockholm, Sweden). 2018;57(1):13–18.
- Cortadellas T, Gascon A, Cordoba O, et al. Surgery improves breast cancer-specific survival in octogenarians with early-stage breast cancer. Int J Surg. 2013;11(7):554–557.
- Cortadellas T, Cordoba O, Gascon A, et al. Surgery improves survival in elderly with breast cancer. A study of 465 patients in a single institution. Eur J Surg Oncol. 2015;41(5):635–640.
- Sun SX, Hollenbeak CS, Leung AM. Deviation from the standard of care for early breast cancer in the elderly: what are the consequences? Ann Surg Oncol. 2015;22(8):2492–2499.
- Derks MGM, on behalf of the EURECCA Breast Cancer Group, Bastiaannet E, Kiderlen M, et al. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: a population-based cohort study from the EURECCA Breast Cancer Group. Br J Cancer. 2018;119(1):121–129.
- Angarita FA, Chesney T, Elser C, et al. Treatment patterns of elderly breast cancer patients at two Canadian cancer centres. Eur J Surg Oncol. 2015;41(5):625–634.
- Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–60.
- Hamaker ME, Bastiaannet E, Evers D, et al. Omission of surgery in elderly patients with early stage breast cancer. Eur J Cancer (Oxford, England: 1990). 2013;49(3):545–552.
- Fang P, He W, Gomez DR, et al. Influence of age on guideline-concordant cancer care for elderly patients in the United States. Int J Radiat Oncol Biol Phys. 2017;98(4):748–757.
- Weggelaar I, Aben KK, Warle MC, et al. Declined guideline adherence in older breast cancer patients: a population-based study in the Netherlands. Breast J. 2011;17(3):239–245.
- Statistical ten-year review. Statistics Denmark; 2012 [cited 2020 Apr 22]. Available from: https://www.dst.dk/Site/Dst/Udgivelser/GetPubFile.aspx?id=16253&sid=sttiaar2012
- Njor SH, Schwartz W, Blichert-Toft M, et al. Decline in breast cancer mortality: how much is attributable to screening? J Med Screen. 2015;22(1):20–27.
- Curigliano G, Burstein HJ, P. Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2019;30(7):1181.
- Christiansen P, on behalf of the Danish Breast Cancer Cooperative Group, Bjerre K, Ejlertsen B, et al. Mortality rates among early-stage hormone receptor-positive breast cancer patients: a population-based cohort study in Denmark. J Natl Cancer Inst. 2011;103(18):1363–1372.
