Abstract
Background: Retrospective studies have suggested that chemotherapy-induced leukopenia is associated with improved recurrence-free or overall survival. The SBG 2000–1 trial was designed to verify the favorable prognosis associated with chemotherapy-induced leukopenia in early breast cancer. Patients not experiencing chemotherapy-induced leukopenia were randomized into standard dosed or individually escalated chemotherapy doses based on the grade of leukopenia after a first standard dose.
Patients and methods: 1452 women in Sweden and Denmark with operable node-positive or high-risk node-negative breast cancer aged 18–60 years were recruited to participate in this trial. Participants received a first FEC cycle at standard doses (600/60/600 mg/m2). Patients (n = 1052) with nadir leukopenia grade 0–2 after the first cycle were randomized between either 6 standard FEC or 6 tailored FEC courses with doses of epirubicin and cyclophosphamide escalated during courses 2 and 3 and thereafter aimed at achieving grade 3 leukopenia. Patients with nadir leukopenia grade 3–4 after the first course continued treatment with standard FEC. Results of the randomized comparison has been published previously. The present study focuses on chemotherapy-induced leukopenia as a covariable with outcome in randomized and non-randomized patients. The prognostic value of leukopenia after course 3, was studied in a Cox model adjusted for cumulative doses of epirubicin and cyclophosphamide. The association of chemotherapy-induced leukopenia with prognosis was a preplanned secondary endpoint for this trial.
Results: The eight-year distant disease-free survival was 73%, 77%, 78% and 83% for patients with leucocyte nadir grade 0, 1, 2 and 3–4, respectively. Higher degree of leukopenia was highly significantly associated to improved distant disease-free survival (HR 0.84, 95% CI 0.74–0.96, p = .008) and overall survival (HR 0.87 (0.76–0.99, p = .032).
Conclusion: This prospective study confirms that chemotherapy-induced leukopenia is a covariable with outcome in primary breast cancer, even after adjustment for chemotherapy doses.
Trial registration number:
Background
Dose calculation of chemotherapy based on body surface area (BSA) has been criticized because it reflects poorly interpatient variability in drug metabolism and may lead to too low or high doses in individual patients [Citation1]. Obese patients are at special risk of being under-dosed when BSA according to common practice is capped to 2.0 m2 instead of using the actual body weight to avoid toxicity [Citation2]. Neutropenia and leukopenia are common dose-limiting side effects of many cytotoxic agents. It has been speculated that chemotherapy-induced leukopenia (CIL) and neutropenia (CIN) may be used as a surrogate marker of chemotherapy efficacy. A large number of retrospective studies have reported that patients experiencing neutropenia or leukopenia have had improved survival [Citation3–25].
The main hypothesis why CIL has a prognostic value has been that CIL reflects the vast interpatient variation in pharmacokinetics, which in turn affects the exposure of both normal- and tumor cells to cytotoxic effects. The covariation of an increasing grade of CIL and CIN and a favorable outcome has been described in several cancer forms including advanced or recurrent ovarian cancer [Citation14,Citation20,Citation24], advanced non-small cell lung cancer [Citation7,Citation10,Citation17], small cell lung cancer [Citation4], early [Citation23] and advanced [Citation6,Citation8,Citation12,Citation22] colorectal cancer, advanced gastric cancer [Citation25], Hodgkin’s lymphoma [Citation26], advanced pancreatic cancer [Citation13], and early breast cancer [Citation3,Citation5,Citation9,Citation11,Citation15,Citation16,Citation19,Citation21]. On the other hand, a few studies have failed to show a statistically significant correlation [Citation27–31]. In a 2012 meta-analysis on breast, lung, gastric, cervical, esophageal, colorectal, ovarian carcinomas or lymphoma the hazard ratio (HR) of death for patients with higher grade leukopenia was 0.69 (95%CI 0.64–0.75) [Citation32]. A summary of studies investigating the relation between treatment outcome of chemotherapy and neutropenia in early breast cancer is shown in . The great majority of the studies showed an association between more severe neutropenia or leukopenia and improved survival [Citation3,Citation5,Citation9,Citation11,Citation15,Citation16,Citation19,Citation21].
Table 1. Publications investigating the prognostic effect of chemotherapy-induced leukopenia or neutropenia in early breast cancer.
In the adjuvant setting, it is of outmost importance to optimize the antitumoral effect of cancer therapy since the patients are treated with a curative intention. Several randomized trials have shown that increased anthracycline doses improved outcome [Citation33–35]. On the other hand, adverse effects of chemotherapy due to overdosing may lead to potentially life-threatening side-effects. SBG 2000–1 is the first adjuvant randomized trial designed to compare individually dosed chemotherapy based on grade of toxicity to standard dosed chemotherapy. Toxicity and efficacy results have been published elsewhere [Citation36,Citation37]. Dose escalation to achieve the optimal level of leukopenia as defined by the trial (grade 3) was feasible and increase in toxicity moderate. Dose escalation did not significantly improve distant-disease or overall survival, but subgroup analyses revealed a significant effect in tumors of histological grade 3. This report focuses on the prognostic effects of chemotherapy-induced leukopenia, which was a secondary endpoint of SBG 2000–1.
Methods
Study design
Between 2001–2003 women in Sweden and Denmark (in Denmark only premenopausal) with early operable node-positive or high-risk node-negative breast cancer aged 18–60 years with ECOG performance status 0–1, normal hematologic function, and no prior malignant diseases, received the first cycle of adjuvant chemotherapy within eight weeks after having undergone complete resection by mastectomy or lumpectomy and axillary node clearance.
The chemotherapy regimen was seven cycles of FEC given every three weeks. Doses for all patients in the first cycle were fluorouracil (F) 600 mg/m2, epirubicin (E) 60 mg/m2, cyclophosphamide (C) 600 mg/m2. Blood counts including white blood cells (WBC) were measured on days 10, 12 or 13, and 15 after cycle 1. After cycles 2–7 WBC nadir was measured on the day following the treatment when the lowest WBC was measured after cycle 1. Patients who had WBC nadir grade 4 after the first cycle received further six cycles with reduced FEC (F 600 mg/m2, E 45 mg/m2 and C 450 mg/m2) and patients with WBC nadir grade 3 received six further cycles of standard FEC. This group was called ‘Registered’ (R) and followed similarly to the randomized groups. Patients with WBC grade 0–2 were randomized between a ‘Standard’ (S) group to receive further six cycles with standard FEC dose level 1 and a ‘Tailored’ (T) group treated with tailored FEC based on the dose escalation scheme prescribed elsewhere [Citation36]. If leukopenia grade 3 was not reached at course 2, doses were escalated one further step at course 3 and continued at the same level for courses 3–7 unless unacceptable toxicity occurred. The maximum doses of E and C was 90 and 1200 mg/m2, respectively. In case of WBC <2.5 × 109/L or platelets <50 × 109/L on day 1 of cycles 2–7, treatment was postponed until these values were reached. In case of WBC nadir grade 4 or febrile neutropenia dose were reduced in subsequent cycles. Primary granulocyte colony-stimulating factor support was not recommended.
Postoperative radiotherapy was administered according to pre-specified regional guidelines. After chemotherapy, patients with hormone receptor positive tumors were to receive adjuvant tamoxifen for five years. For postmenopausal women aromatase inhibitors were allowed after an amendment in April 2006. All endocrine therapies were according to regional and national guidelines.
The study was conducted in accordance with the Declaration of Helsinki. The patients received oral and written information, and a written informed consent was obtained from all patients. The study was approved by the ethical committees with jurisdiction for the participating institutions
Statistical considerations
Predictive factors for the development of treatment induced leukopenia were tested with both course 1 and 3 as endpoints, since course one was given at identical doses in all patients, while leukopenia after course 3 was tested in survival analyses. A sensitivity analysis was done to exclude the possibility that the choice of course 3 instead of any other course would have resulted in a false positive result. In these analyses we analyzed the prognostic impact of mean WBC nadir grade after courses 3–7 and distant-disease free survival with cox regression analysis.
The analysis of the relation between CIL and prognosis was based on leukopenia after course 3 when the chemotherapy dose of all patients had been finally adjusted. Cumulative chemotherapy doses of E and C were used as covariates in addition to grade of leukopenia. Grade 3 and 4 were combined and coded as leukopenia grade 3, since following doses were reduced for the 33 patients (2.3%) who experienced grade 4 leukopenia at course 3.
The association between demographics and disease characteristics and leukocyte nadir grade was tested with Mann–Whitney test, except for BMI, age and tumor size where Spearman´s rank correlation test was used. Distant disease-free survival (DDFS) and overall survival (OS) were estimated by the Kaplan–Meier method and comparisons between different leukocyte nadir groups were done by adjusted Cox regression analysis.
Results
One thousand five hundred forty-five patients were enrolled in the study. Of these 82 were excluded from the intention to treat analysis, leaving 1453 patients, for details see Lindman et al [Citation37]. One further patient was excluded from the present study, because of missing information on BSA, leaving 1452 patients, 401 in the registered, 528 in the standard and 523 in the tailored FEC groups. Seventy-two patients had missing nadir leukocytes values at course 3 leaving 1380 for survival analyses (381, 499 and 500 patients in the R, S and T groups, respectively).
Baseline patient characteristics and their association to leukopenia after course 3 are shown in and after course 1 in Supplementary Table 1. Lean patients (lower BMI) experienced significantly more leukopenia (p < .001). There was a weak but significant association between higher age at diagnosis and larger tumor size and more severe leukopenia. As expected, there was a highly significant association between cumulative doses of E and C and leukopenia at course 3.
Table 2. Demographic and baseline disease characteristics according to leukocyte nadir grade after course 3 (n = 1380).
Eight-year DDFS rate were 73%, 77%, 78% and 83% for leukocyte nadir grade 0, 1, 2 and 3–4 and OS rate 77%, 79%, 81%, 86% respectively. Cox regression analysis of DDFS according to leukopenia grade after course 3 is shown in . The result was highly significant with a HR for grade of leukopenia of 0.84 (95% CI 0.739–0.955, p = .008). The cumulative dose of E or C did not significantly affect DDFS (HR 0.96, 95% CI 0.93–1.014 and 1.002, 95% CI 1.00–1.005, respectively). A Kaplan Meier curve for DDFS according to different leukopenia grades and tailored group separately is shown in . A sensitivity analysis testing mean nadir grade during course 3 to 7 was highly significant, HR 0.81 (95% CI 0.699–0.947, p = .008). Subgroup analyses of the association between leukopenia and DDFS are shown in . Patients with grade 3 tumor had a significantly stronger association between leukopenia and DDFS (HR 0.764, 95% CI 0.648–0.901, p < .001), and a test of interaction between grade and leukopenia was significant (p = .031). The association between leukopenia at course 3 and OS was significant, HR 0.87 (95% CI 0.76–0.99, p = .032), this is shown in .
Figure 1. Kaplan Meier curves for distant disease-free survival (DDFS) according to leukocyte nadir grades 0-3 after course 3 in standard and registered arms, tailored arm (T) separately in black.
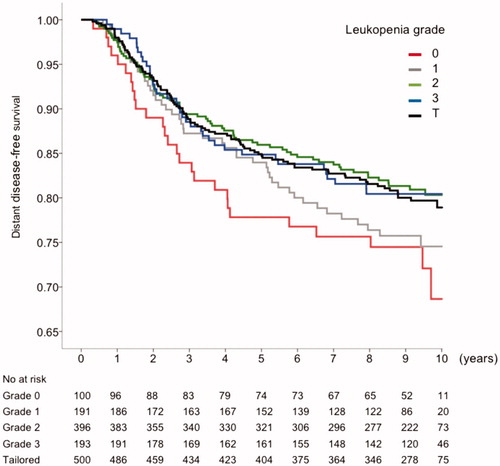
Figure 2. A forest plot with the Hazard ratio and P-value for interaction in different subgroups regarding the correlation between DDFS and leukopenia after the third course. Red thin line indicated the HR in the whole population. BMI = mass index, PreDK = Danish premenopausal patients, PreSE = Swedish premenopausal patients, PostSE = Swedish postmenopausal patients, T ≤ 2= tumor diameter 2 cm or less, tumor diameter over 2 cm, N: nodal status; ER: estrogen receptor status; G: tumor grade; HER2: HER2 status.
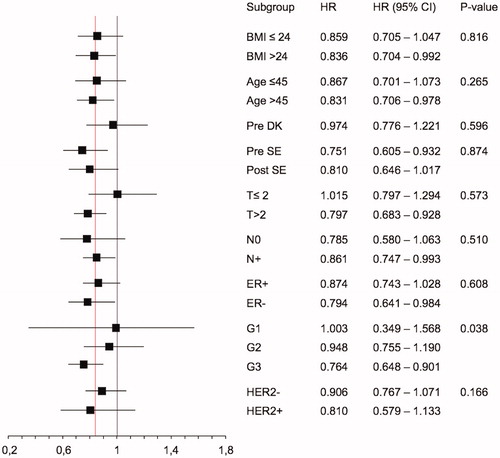
Table 3. Cox regression analysis of DDFS and OS, leukocyte nadir after the third course, cumulative doses of epirubicin and cyclophosphamide.
Discussion
The SBG 2000–1 study was designed as the first randomized controlled trial prospectively studying the prognostic value of CIL and the effect on outcome by tailoring chemotherapy dosing according to leukopenia grade compared with standard BSA-based dosing in early breast cancer with a low grade of leukopenia.
The hypothesis was based on the observation from two retrospective studies on the relationship between leukopenia and prognosis after adjuvant chemotherapy of breast cancer published 20 years ago [Citation19,Citation21]. The randomized part of the SBG 2000–1 has been published elsewhere [Citation37]. In the present report we focus in more detail on the association between leukopenia grade and outcome and our main finding that increasing grade of leukopenia, but not chemotherapy dose, was associated with significant improvements of DDSF and OS, HR 0.84 and 0.87 respectively.
We also wanted to summarize the literature on leukopenia during adjuvant chemotherapy as a prognostic factor for long term outcome in early breast cancer. To our knowledge, no previous systematic review of this matter have been published lately. We found nine published retrospective studies of the relationship between CIL and the prognosis of early breast cancer, for a summary see . Leukopenia or neutropenia was associated to improved outcome in eight out of nine studies. In the largest of these studies chemotherapy related toxicities were collected from 6248 patients with early stage breast cancer from four controlled trials with several different chemotherapy regimens [Citation3]. Information on neutropenia was available for 5886 patients and the analysis showed that neutropenia grade was an independent predictor for relapse free survival (HR 0.86; 95% CI 0.76–0.97; p = .02). A similar trend was seen for breast cancer specific survival (HR 0.87; 95% CI 0.75–1.01; p = .06) [Citation3]. Two studies [Citation9,Citation15] were based on patients treated with the FEC regimen, the same as our study used. Both these studies indicated that CIL/N is significantly associated to improved outcome. The overall findings in earlier retrospective studies, with eight out of nine showing a prognostic value of CIL or CIN in early breast cancer, are confirmed by the results of the SBG 2000–1 study. In metastatic breast cancer data are scarce, Koutras and coworkers could not find any favorable impact of CIN on treatment efficacy, not even after adjustment for the use of G-CSF [Citation29].
One strength of the present study design, apart from being prospective, is the elaborate method of capturing the true leukocyte nadir by measuring leukocytes on days 10, 12 or 13, and 15 after the first cycle. At subsequent cycles nadir was measured once on the cycle-day that nadir occurred after cycle 1.
The impact of leukopenia on DDFS was consistent throughout most subgroups, however, the association was more pronounced in grade 3 tumors, indicating that achievement of leukopenia may be more important in aggressive cancers.
In this study, women with high BMI experienced less severe neutropenia than those with low/medium BMI, even though BSA was not capped at 2.0 m2 when doses were calculated. This finding has also been reported previously [Citation38] and implies that obese patients are at risk of being under-dosed which in turn can lead to suboptimal efficacy. It is reasonable to believe that this risk increases if BSA is capped. It has been speculated that factors related to obesity such as pharmacokinetic differences [Citation39] and comorbidities [Citation40] could contribute to chemotherapy intolerance for this group. Since patients with high BMI had less high-grade leukopenia in our study, surveillance with blood tests and dose tailoring could be of increased importance for this group. The recently published Panther study, coordinated from the Karolinska Institute, combined haematologically based tailoring of 4 cycles of EC and 4 docetaxel (D) cycles with dose dense scheduling, supported with G-CSF, the comparator was standard chemotherapy with 3 FEC and 3 D. The more aggressive regimen benefited obese patients more than non-obese [Citation41] without increased problems with tolerance.
Data on capecitabine, that is an oral fluoropyrimidine used to treat several types of cancer including breast cancer, also support the correlation between increased toxicity and higher efficacy.
It’s main dose limiting toxicities are hand-foot syndrome (HFS) and diarrhea. There is evidence that patients experiencing HFS have a better prognosis than patients without this toxicity [Citation42]. Interestingly, an association between more toxicity and higher efficacy has also been reported on targeted drugs like sunitinib in renal cell cancer with neutropenia and hypertension [Citation43–45] but not when combined with docetaxel in metastatic breast cancer [Citation46]. In colorectal cancer, rash has been reported to associate with better prognosis during treatment with EGFR inhibitors [Citation47].
The randomized comparison in the main publication [Citation37] failed to demonstrate superiority of dose tailored (i.e. escalated) FEC-chemotherapy over standard BSA-based FEC [Citation37], but there was still a numerical advantage for tailored therapy with the DDFS point estimate of 0.87 (95% CI 0.67–1.14). In addition, 27% of patients achieved high leukopenia grade with the starting dose (F 600 mg/m2, E 60 mg/m2 and C 600 mg/m2) and, vice versa, 10% of those in the tailored arm did not achieve G3(-4) leukopenia. It is reasonable to believe that the SBG 2000–1 was under-powered. Recently an EBCTCG meta-analysis has demonstrated the importance of chemotherapy dose intensity in primary breast cancer [Citation48]. Increasing the dose intensity by either shortening treatment intervals or giving individual doses sequentially rather than concomitantly, which allowed higher doses, improved both recurrence-free (RR 0·86, 95% CI 0·82–0·89) and overall survival (RR 0·87, 95% CI 0·83–0·91; p < .0001). The increased efficacy was seen both with anthracycline regimens with and without taxanes. Several individual randomized trials have shown the importance of anthracycline dose and dose intensity [Citation33–35,Citation49], while the role of dose intensity of cyclophosphamide is less clear. Two large trials, NSABG-22 and NSABG-25 did not show dose escalation of cyclophosphamide above standard doses to be beneficial [Citation50,Citation51].
Our study started before the adjuvant taxane containing regimens were introduced. Since more than a decade, docetaxel (D) and paclitaxel (P) are a standard component of adjuvant chemotherapy for breast cancer [Citation52]. D is the most used taxane in the Scandinavian countries. Leukopenia-based dosing of D instead of an anthracycline based regimen, is probably more difficult because of non-haematological toxicity and the fact that the commonly used dose of 100 mg/m2 iv q 3w [Citation52], induces leukopenia/neutropenia of G 3–4 in virtually, all patients. Thus, grade of leukopenia is not a useful discriminator of individual toxicity. P, the other widely used taxane, is preferably given at the dose of 80 mg/m2 iv weekly [Citation52] since this schedule is more efficacious and less myelotoxic than q3 weekly administration. Weekly P gives rise to little leuko- and neutropenia which means that this widely used regimen is not suitable for dosing based on grade of leukopenia either. The most common dose limiting toxicity for weekly paclitaxel is peripheral neuropathy, which is difficult to utilize for toxicity-based dosing, since it appears late, is cumulative, and often worsens even long after treatment discontinuation.
The previously mentioned Panther study, however, indicated that dose tailoring is possible also with taxane regimens [Citation53]. The dose-dense and tailored chemotherapy was not statistically significantly superior to standard treatment with reference to the primary endpoint, recurrence free survival (p = .06), but the HR of 0.79 (95% CI 0.61–1.01), was even lower than the estimated benefit of dose intensification in the EBCTCG meta-analysis (0.86) [Citation48]. The Panther trial also demonstrated that dose tailoring according to leukopenia is possible in a taxane regime; the patients in the tailored group received 10%, 37%, and 6% higher total doses of E, C and D respectively compared to the initial dose due to dose escalation of EC and D courses 2–4 according to haematological tolerance. Would it be possible to implement the concept of leukopenia/neutropenia-based dosing of chemotherapy? With flat doses large proportion of patients are either under-dosed or over-dosed. This might be avoided by leukopenia-based dosing. A downside of this strategy is the need of collecting more blood samples which can be inconvenient for the patient. Another problem is the increased use of routinely administered G-CSF support, which may complicate dosing. According to EORTC guidelines for the use of G-CSF in adult cancer patients G-CSF is recommended in patients receiving a chemotherapy regimen with a high risk (≥20%) for febrile neutropenia, which includes the commonly used anthracycline taxane based combinations in the adjuvant therapy of breast cancer [Citation54]. According to a recent review the of prophylactic use G-CSF may not be a cost-benefit strategy for all adjuvant chemotherapy regimens used in the adjuvant treatment of breast cancer, possible with the exception of sequential docetaxel and anthracycline treatment if prophylactic treatment is limited to the docetaxel part [Citation55]. In addition, if the tailoring of doses is aiming at increasing the dose to the level of tolerance, non-haematological toxicity can increase [Citation56]. We think that some of the experiences from our study are applicable in every day practise given the fact that 27% of the patients reached haematological tolerance limit already with the doses relatively low in the present trial compared to current dose recommendations.
The notion that chemotherapy-induced leuko-/neutropenia can predict outcome has until now been based on retrospective data only. The present prospective study verifies the prognostic importance of CIL and consequently, gives support to the second part of our hypothesis, that tailored dosing of chemotherapy aiming at achieving an optimal leukopenia may increase efficacy.
Supplemental Material
Download MS Word (16.5 KB)Acknowledgments
The authors want to thank study nurse Wiviann Björklund for excellent administrative work and communication with patients, and study nurses and researchers in all the patient recruiting centers.
Disclosure statement
The authors have no potential conflicts of interest to declare.
References
- Gurney H. How to calculate the dose of chemotherapy. Br J Cancer. 2002;86(8):1297–1302.
- Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. JCO. 2003;21(24):4524–4531.
- Abraham JE, Hiller L, Dorling L, et al. A nested cohort study of 6,248 early breast cancer patients treated in neoadjuvant and adjuvant chemotherapy trials investigating the prognostic value of chemotherapy-related toxicities. BMC Med. 2015;13(1):306.
- Banerji U, Ashley S, Coward J, et al. The association of chemotherapy induced neutropenia on treatment outcomes in small cell lung cancer. Lung Cancer. 2006;54(3):371–377.
- Cameron DA, Massie C, Kerr G, et al. Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer. 2003;89(10):1837–1842.
- Chen Y, Wang Y, Shi Y, et al. Timing of chemotherapy-induced neutropenia predicts prognosis in metastatic colon cancer patients: a retrospective study in mFOLFOX6 -treated patients. BMC Cancer. 2017;17(1):242.
- Di Maio M, Gridelli C, Gallo C, et al. Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol. 2005;6(9):669–677.
- Hamauchi S, Yamazaki K, Masuishi T, et al. Neutropenia as a predictive factor in metastatic colorectal cancer treated with TAS-102. Clin Colorectal Cancer. 2017;16(1):51–57.
- Han Y, Yu Z, Wen S, et al. Prognostic value of chemotherapy-induced neutropenia in early-stage breast cancer. Breast Cancer Res Treat. 2012;131(2):483–490.
- Huang CS, Liu L, Liu J, et al. Association of chemotherapy-induced leucopenia with treatment outcomes in advanced non-small-cell lung cancer cases receiving the NP regimen. Asian Pac J Cancer Prev. 2012;13(9):4481–4485.
- Ishitobi M, Komoike Y, Motomura K, et al. Prognostic significance of neutropenia on day one of anthracycline-based neoadjuvant chemotherapy in operable breast cancer. Oncology. 2010;78(3-4):213–219.
- Kasi PM, Kotani D, Cecchini M, et al. Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer. 2016;16(1):467.
- Kurihara T, Kogo M, Ishii M, et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with unresectable pancreatic cancer. Cancer Chemother Pharmacol. 2015;76(6):1217–1224.
- Lee CK, Gurney H, Brown C, et al. Carboplatin-paclitaxel-induced leukopenia and neuropathy predict progression-free survival in recurrent ovarian cancer. Br J Cancer. 2011;105(3):360–365.
- Ma RM, Chen CZ, Zhang W, et al. Prognostic value of chemotherapy-induced neutropenia at the first cycle in invasive breast cancer. Medicine (Baltimore). 2016;95(13):e3240.
- Mayers C, Panzarella T, Tannock IF. Analysis of the prognostic effects of inclusion in a clinical trial and of myelosuppression on survival after adjuvant chemotherapy for breast carcinoma. Cancer. 2001;91(12):2246–2257.
- Pallis AG, Agelaki S, Kakolyris S, et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with advanced non-small cell lung cancer treated with front-line docetaxel-gemcitabine chemotherapy. Lung Cancer. 2008;62(3):356–363.
- Paridaens R, Wildiers J, Dumez H, et al., editors. Impact of dose-intensity of adjuvant CMF on disease-free (DFS) and overall survival (OS) in breast cancer (BC): a retrospective analysis. 27th ESMO Congress. 18–22 October; 2002; Nice: Oxford university press.
- Poikonen P, Saarto T, Lundin J, et al. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer. 1999;80(11):1763–1766.
- Rocconi RP, Matthews KS, Kemper MK, et al. Chemotherapy-related myelosuppression as a marker of survival in epithelial ovarian cancer patients. Gynecol Oncol. 2008;108(2):336–341.
- Saarto T, Blomqvist C, Rissanen P, et al. Haematological toxicity: a marker of adjuvant chemotherapy efficacy in stage II and III breast cancer. Br J Cancer. 1997;75(2):301–305.
- Shitara K, Matsuo K, Takahari D, et al. Neutropaenia as a prognostic factor in metastatic colorectal cancer patients undergoing chemotherapy with first-line FOLFOX. Eur J Cancer. 2009;45(10):1757–1763.
- Sunaga T, Suzuki S, Kogo M, et al. The association between neutropenia and prognosis in stage III colorectal cancer patients receiving adjuvant chemotherapy. Eur J Cancer Care (Engl). 2014;23(3):394–400.
- Tewari KS, Java JJ, Gatcliffe TA, et al. Chemotherapy-induced neutropenia as a biomarker of survival in advanced ovarian carcinoma: an exploratory study of the gynecologic oncology group. Gynecol Oncol. 2014;133(3):439–445.
- Yamanaka T, Matsumoto S, Teramukai S, et al. Predictive value of chemotherapy-induced neutropenia for the efficacy of oral fluoropyrimidine S-1 in advanced gastric carcinoma. Br J Cancer. 2007;97(1):37–42.
- Klimm B, Reineke T, Haverkamp H, et al. Role of hematotoxicity and sex in patients with Hodgkin’s lymphoma: an analysis from the German Hodgkin Study Group. JCO. 2005;23(31):8003–8011.
- Jereczek-Fossa B, Jassem J, Karnicka-Mlodkowska H, et al. Does chemotherapy-induced leukopenia predict a response in small-cell lung cancer? J Cancer Res Clin Oncol. 1998;124(2):106–112.
- Kim JJ, Park JY, Kim DY, et al. Is chemotherapy-induced neutropenia a prognostic factor in patients with ovarian cancer? Acta Obstet Gynecol Scand. 2010;89(5):623–628.
- Koutras AK, Hellenic Cooperative Oncology Group, Fountzilas G, Dafni U, et al. Myelotoxicity as a prognostic factor in patients with advanced breast cancer treated with chemotherapy: a pooled analysis of two randomised trials conducted by the Hellenic Cooperative Oncology Group. Anticancer Res. 2008;28(5B):2913–2920.
- Kumpulainen EJ, Hirvikoski PP, Johansson RT. Neutropenia during adjuvant chemotherapy of breast cancer is not a predictor of outcome. Acta Oncol. 2009;48(8):1204–1206.
- Smoragiewicz M, Javaheri KR, Yin Y, et al. Neutropenia and relative dose intensity on adjuvant FOLFOX chemotherapy are not associated with survival for resected colon cancer. J Gastrointest Canc. 2014;45(4):460–465.
- Shitara K, Matsuo K, Oze I, et al. Meta-analysis of neutropenia or leukopenia as a prognostic factor in patients with malignant disease undergoing chemotherapy. Cancer Chemother Pharmacol. 2011;68(2):301–307.
- Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90(16):1205–1211.
- de Azambuja E, Paesmans M, Beauduin M, et al. Long-term benefit of high-dose epirubicin in adjuvant chemotherapy for node-positive breast cancer: 15-year efficacy results of the Belgian multicentre study. JCO. 2009;27(5):720–725.
- French Adjuvant Study Group. Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol. 2001;19(3):602–611.
- Edlund P, Ahlgren J, Bjerre K, et al. Dose-tailoring of FEC adjuvant chemotherapy based on leukopenia is feasible and well tolerated. Toxicity and dose intensity in the Scandinavian Breast Group phase 3 adjuvant Trial SBG 2000-1. Acta Oncol. 2011;50(3):329–337.
- Lindman H, Andersson M, Ahlgren J, et al. A randomised study of tailored toxicity-based dosage of fluorouracil-epirubicin-cyclophosphamide chemotherapy for early breast cancer (SBG 2000-1). Eur J Cancer. 2018;94:79–86.
- Poikonen P, Blomqvist C, Joensuu H. Effect of obesity on the leukocyte nadir in women treated with adjuvant cyclophosphamide, methotrexate, and fluorouracil dosed according to body surface area. Acta Oncol. 2001;40(1):67–71.
- Sparreboom A, Wolff AC, Mathijssen RH, et al. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. JCO. 2007;25(30):4707–4713.
- Haslam DW, James WP. Obesity. Obesity. Lancet. 2005;366(9492):1197–1209.
- Matikas A, Foukakis T, Moebus V, et al. Dose tailoring of adjuvant chemotherapy for breast cancer based on hematologic toxicities: further results from the prospective PANTHER study with focus on obese patients. Ann Oncol. 2019;30(1):109–114.
- Zielinski C, Lang I, Beslija S, et al. Predictive role of hand-foot syndrome in patients receiving first-line capecitabine plus bevacizumab for HER2-negative metastatic breast cancer. Br J Cancer. 2016;114(2):163–170.
- Donskov F, Michaelson MD, Puzanov I, et al. Sunitinib-associated hypertension and neutropenia as efficacy biomarkers in metastatic renal cell carcinoma patients. Br J Cancer. 2015;113(11):1571–1580.
- Fujita T, Wakatabe Y, Matsumoto K, et al. Leukopenia as a biomarker of sunitinib outcome in advanced renal cell carcinoma. Anticancer Res. 2014;34(7):3781–3787.
- Rautiola J, Donskov F, Peltola K, et al. Sunitinib-induced hypertension, neutropaenia and thrombocytopaenia as predictors of good prognosis in patients with metastatic renal cell carcinoma. BJU Int. 2016;117(1):110–117.
- Bergh J, Mariani G, Cardoso F, et al. Clinical and pharmacokinetic study of sunitinib and docetaxel in women with advanced breast cancer. Breast. 2012;21(4):507–513.
- Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–2048.
- EBCTCG. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393(10179):1440–1452.
- Fumoleau P, Kerbrat P, Romestaing P, et al. Randomized trial comparing six versus three cycles of epirubicin-based adjuvant chemotherapy in premenopausal, node-positive breast cancer patients: 10-year follow-up results of the French Adjuvant Study Group 01 trial. JCO. 2003;21(2):298–305.
- Fisher B, Anderson S, DeCillis A, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-25. JCO. 1999;17(11):3374–3388.
- Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-22. JCO. 1997;15(5):1858–1869.
- Bachegowda LS, Makower DF, Sparano JA. Taxanes: impact on breast cancer therapy. Anticancer Drugs. 2014;25(5):512–521.
- Foukakis T, for the Swedish Breast Cancer Group (SweBCG), the German Breast Group (GBG), and the Austrian Breast & Colorectal Cancer Study Group (ABCSG), von Minckwitz G, Bengtsson NO, et al. Effect of tailored dose-dense chemotherapy vs standard 3-weekly adjuvant chemotherapy on recurrence-free survival among women with high-risk early breast cancer: a randomized clinical trial. JAMA. 2016;316(18):1888–1896.
- Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
- Younis T, Rayson D, Jovanovic S, et al. Cost-effectiveness of febrile neutropenia prevention with primary versus secondary G-CSF prophylaxis for adjuvant chemotherapy in breast cancer: a systematic review. Breast Cancer Res Treat. 2016;159(3):425–432.
- Palmieri C, Jones A. The 2011 EBCTCG polychemotherapy overview. Lancet. 2012;379(9814):390–392.
