Abstract
Purpose: Cure- and toxicity rates of prostate IGRT can both be affected by ill-chosen planning target volume (PTV) margins. For dose-escalated prostate radiotherapy, we studied the potential for organ at risk (OAR) sparing and compensation of prostate motion with robust plan optimization using the coverage probability (CovP) concept compared to conventional PTV-based IMRT.
Material and methods: We evaluated plan quality of CovP-plans for 27 intermediate risk prostate cancer patients treated in a prospective study (78 Gy/39 fractions). Clinical target volume (CTV) and OARs were contoured on three separate CTs to capture movement and deformation. To define the internal target volume (ITV), the union of CTV1-3 was encompassed by an isotropic margin of 7 mm for the planning process. CovP-dose distribution is optimized considering weight factors for IMRT constraints derived from probabilities of systematic organ displacement in the three CTs. CovP-dose volume histograms (DVHs) were compared with additionally calculated conventional PTV-based IMRT plans. PTV-based IMRT was planned on one-single CT with an isotropically expanded CTV to generate the PTV (i.e., CTV1 + 7mm) and was evaluated on the two other CTs.
Results: The CovP-concept showed higher robustness in target volume coverage. Target miss was frequently observed with PTV-based IMRT, resulting in cold spots until 70 Gy with the CovP-concept. The target dose at 74 Gy was comparable, while further the dose–escalation (75–78 Gy) was improved with PTV-based IMRT. However, dose–escalation with PTV-based IMRT was associated with increased OAR-doses, especially in high-dose areas.
Conclusions: Probabilistic dose-escalated IMRT was feasible in this prospective study. Comparison of the CovP-concept with PTV-based IMRT revealed superiority with regard to target-coverage and sparing of OARs. The CovP-concept implements a robust plan optimization strategy for organ deformation and motions and could, therefore, serve as a less demanding compromise on the way to adaptive IGRT avoiding daily time-consuming re-planning.
SUMMARY
We evaluated the robustness of coverage probability (CovP)-based IMRT plans within a prospective study for prostate cancer radiotherapy. The treatment plans were compared with newly calculated conventional PTV-based IMRT plans. We were able to show that CovP led to a clearly more robust target coverage by avoiding hot spots at OARs compared to conventional PTV-based IMRT. In addition, negative consequences of an inflated PTV can be ameliorated by a more relaxed CovP-based dose prescription.
Introduction
Intensity-modulated radiotherapy (IMRT) became standard of care for dose-escalated radiotherapy (RT) of prostate cancer (PC) [Citation1–5]. Conventional IMRT is planned on a single computed tomography (CT) for dose calculation. A fixed margin is then added to the clinical target volume (CTV) to account for set-up and internal motion uncertainties and the dose is prescribed for the entire planning target volume (PTV). Optimal dose delivery to the target volume by avoiding toxicity of organs at risk (OAR) can be achieved by different strategies. Dose–escalation can be realized by a shrinking field boost i.e., reducing the PTV margins toward the rectum down to 0 cm to avoid gastrointestinal (GI) toxicity. Image-guidance using fiducials and reduced margins for the PTV represents another technique to reduce toxicity by a smaller overlap of target volume with OARs. The smaller the margin and consequently the OAR-overlap, the smaller the compensation of uncertainties. Minimized margins might also lead to target miss i.e., to loss of tumor control [Citation6]. However, rigorous prescription of a PTV-concept is not common practice, especially when OAR-constraints are exceeded. Usually underdosages in PTV are accepted to meet constraints and to avoid increased toxicity rates.
Storey et al. could identify that a key indicator of RTOG G2+-late toxicity is the percentage volume of the rectum receiving doses above 70 Gy (V70 > 25%) [Citation7]. The expected increased rectal toxicity of dose–escalation was the rationale for the development of a probabilistic RT-concept for prostate cancer [Citation8–10]. Patients with higher mobility of the pelvic organs and form-variable prostate might benefit from this method [Citation11,Citation12]. On the other hand, a dose–escalation to 78 Gy in a larger volume using the target volumes of 3 CTs could potentially also lead to more side effects.
Therefore, we investigated in a prospective study the potential of the pre-adaptive, robust optimization coverage probability (CovP) concept in prostate cancer as developed by Baum et al. [Citation11,Citation12]. In short, the CovP algorithm optimizes treatment plans by sparing OARs and compensating for target movements or deformation, depending on individual spatial probabilities of the presence of an OAR or the target. Contours of three planning CTs are used for dose–calculation and sparing of OARs, depending on individual deformation/filling changes in contrast to PTV-based IMRT derived from only one CT (Citation11,Citation12). Plan quality, robustness of treatment plans and achievement of constraints were evaluated for both treatment concepts to estimate potential benefits of each concept.
Material and methods
Study design
A prospective observational study on dose-escalated IMRT was conducted at the University of Tübingen with the CovP-concept as described by Baum et al. (Citation11,Citation12). The study was approved by the institutional review board (257/2007B01).
Patients
Twenty-eight patients were treated within this prospective study. Inclusion criteria comprised histopathological confirmed adenocarcinoma of the prostate, age ≤85 years, Karnofsky performance state (KPS) ≥70%, localized disease (bone scan, abdominal/pelvic CT), intermediate risk according to D’Amico classification and informed consent of the patient. Exclusion criteria were prior prostatectomy or prior transurethral resection, prior pelvic radiotherapy, other malignancies or severe diseases (e.g., decompensated heart insufficiency, chronic inflammatory bowel disease, blood coagulation restrictions) or mental restrictions impairing participation in a clinical trial. According to national guidelines, short term androgen deprivation therapy was recommended for 6 months (Citation4).
Treatment planning and CovP-concept
Three planning CTs (SOMATOM Sensation 64, Siemens Healthcare, Erlangen, Germany) were performed on consecutive days using a rectum- and bladder protocol (Supplement). The three planning CTs were registered rigidly using mutual information based on bony anatomy (Oncentra Masterplan® (Theranostic GmbH, Solingen, Germany)) and CTVs/OARs (bladder, rectum and femoral heads) were contoured in each planning CT ().
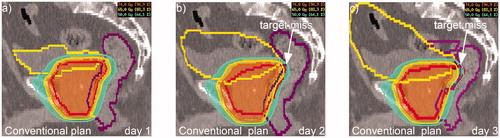
Figure 1. PTV-based IMRT plan. A conventional IMRT plan (i.e., cPTV1) was generated and demonstrated with cPTVs and OARs on day1-3 (a-c) indicating target miss at day 2-3 (b-c).
The merged CTVs 1-3 (CTVunion) were encompassed by an isotropic 3 D-safety margin of 7 mm to define the internal target volume (CovP-ITV). The same margin was added to the OARs bladder and rectum creating the planning OAR volumes (PRV). The CovP-concept employs spatially variable weight factors for the target- and OAR-cost functions that allow the optimizer to consider the likelihood of finding the CTV/OAR in a given point (with Hyperion® version 2.2.5 and 2.3, University of Tübingen, Germany, as the planning software, ). The variable weight factors were derived from the probabilities of systematic organ displacement, as assessed from the repeated CTs, and systematic 3 D-setup errors, described by a 3 D Gaussian distribution. Class solutions were applied for RT-planning containing EUD (equivalent uniform dose)-defined constraints for OARs (EUD rectum: 65 Gy with a serial complication mechanism (h(d)=dk with volume effect parameter k = 12.0; EUD bladder: 60 Gy with k = 8) (Citation11). In addition, EUD-defined target volume cost functions (target EUD: 78 Gy) and standard angles for step and shoot IMRT (usually 20°, 60°, 100°, 140°, 220°, 260°, 300° and 340°) were applied (Citation11,Citation12). All patients were treated with step-and-shoot IMRT to a total dose of 78 Gy in 39 fractions with 15 MV-photons (linear accelerator (Elekta Synergy S, Elekta Oncology Systems®, Crawley, UK)). The verification strategy and an overview of the different strategies are given in the supplement.
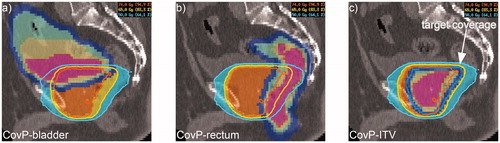
Figure 2. CovP-IMRT plans. The CovP-plan was calculated based on three consecutive planning CTs implementing deformation and movements of OAR and CovP-ITV. The CovP is demonstrated for bladder (a), rectum (b) and the ITV (c). Coverage probability is visualized by colors (pink = three times, yellow = twice, green = once). Blue demonstrates the safety margin of 7mm for CovP-ITV and OARs.
PTV-based treatment vs. CovP-concept
Conventional PTV-based radiotherapy is performed using one planning CT. To define a PTV-based treatment plan, we used the structures of the first planning CT and added an isotropic margin of 7 mm to define the conventional PTV (cPTV) in all datasets. cPTV-based IMRT-plans were generated with XXX. Twenty-seven cPTV-plans were calculated using only cPTV1 and respective OARs of CT1 (i.e., bladder, rectum, femoral heads). In addition, CTV2 and 3 were expanded by an isotropic margin of 7 mm to define cPTV2 and cPTV3. PTV-based IMRT-plans (cPTV1) were optimized using the same class solution, OAR-/target-constraints and treatment technique as applied to the CovP-plans to ensure the same plan quality. To measure the plan quality and robustness of both plan concepts toward organ movement, we compared dose volume histogram (DVH) statistics using a hybrid DVH (mean) for OARs and target volumes. A graphic and tabular comparison of the DVHs of the applied CovP-plans with the PTV-based plans was carried out. To compare the CovP-plan (based on 3 CTs) with a cPTV-based plan, a hybrid-DVH of the three mean DVH of each pCT (bladder 1-3, rectum 1-3) was calculated. The functional CovP-ITV was compared with the mean DVH over three cPTVs (cPTV1-3).
Statistical analysis
Microsoft Excel 2010 (Version 14.0.7145.5000) was used for the descriptive statistics. SPSS (Statistical Package for Social Sciences ‘IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, N.Y., USA)) was used for paired t-tests and Bonferroni correction of DVH-parameters V60-V80 (Vx = Volume receiving ≥ x Gy). The confidence interval was set at 95% and the significance level at p < 0.05.
Results
From 2008 to 2010, twenty-eight patients (median age 70) with intermediate-risk prostate cancer were enrolled in this prospective study. Dose-escalation to 78 Gy was feasible in all patients. The coverage probability concept was realized in 27 patients. Study outcome and details of toxicity were reported elsewhere (Citation13).
Robustness of target coverage
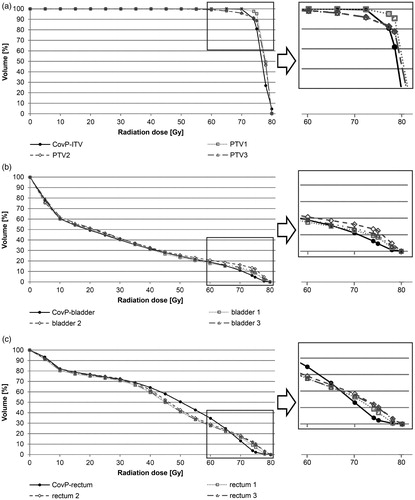
DVH-statistics of mean cPTV1-3 and the weighted CovP-ITV are presented in and Supplement 1. As expected, cPTV1 was the best plan at day 1, but worse at day 2-3 regarding target coverage at lower radiation doses. Significant target miss was observed from 60-70Gy (p < 0.01, Supplement Table 1). The volume of target miss reached ∼1-3% of the cPTV at 65-70 Gy (mean cPTV1-3: V65: 98.7%, V70: 97.2%) compared to the CovP-plan covering 100% at 65 Gy and 99.7% at 70 Gy. The 95%-isodose (V74) was comparable and within acceptable limits for both plans (CovP-ITV: 90.4% and mean cPTV1-3: 93.5%; p = 0.364, Supplement Table 1). Regarding high-dose regions, mean cPTV1-3 was more advantageous from 75-78Gy (p < 0.01). The weighted CovP-ITV-plan achieved 78 Gy in 26.8% compared to the conventional PTV-based plan with 47.4%. However, maximal radiation doses of 80 Gy were more pronounced in the CovP-plan (p < 0.01). A comparison of relative CTV (as well as bladder- and rectum) DVH-parameters is given in Supplement Tables 4-6.
Bladder sparing
A comparison of DVH-statistics of mean bladder1-3 and the weighted PRV_CovP-bladder (CovP-bladder) is shown in (, Supplement Table 2). Both plans led to equal dose distributions in the bladder from V0-65. The CovP-plan demonstrated a significantly better bladder sparing from V74- V78 (p = 0.01). In the CovP-concept, V74/V78 is 6.2%/0.8% compared to 11.2%/3.4% in the PTV-based plan. The absolute radiation dose differences amounted to 5.1 Gy at V74, 5.5 Gy at V75 and 2.4 Gy at V78. The V80 was below 0.1 Gy in both plans.
Rectal sparing
A comparison of DVH-statistics of mean rectum_1-3 and the weighted planning OAR volume PRV_CovP-rectum (CovP-rectum) is shown in and Supplement Table 2. Significant differences between both plans were observed for V60 and V70-78. The DVH-curves were comparable up to V35. Better rectal sparing was seen from V40-60 for the cPTV-plan, while given constraints were met in both plans (V40 < 80%, V60 < 35%). Absolute differences between CovP- and cPTV-plans were 3.6% for V40 and 6.7% for V60. However, high-dose areas received significantly lower radiation doses with the CovP-plan. Rectal V70 was 4.3% lower in the CovP-plan (13.0 vs. 17.3%) meeting the constraint of V70 < 15% in contrast to the cPTV-plan. The same was shown for V74-78, with significant reductions of 7.1%, 6.7% and 1.8 (V74: 3.7% vs. 10.8%; V75: 2.2% vs. 8.9%; V78: 0.3% vs. 2.1%). The V80-values were ∼0% in both plans.
Discussion
In this prospective study, we compared a conventional PTV-based strategy that requires no patient-specific a-priori information with a CovP-concept that includes patient-specific information about organ motion. While the cPTV-based strategy compensates for all uncorrected errors by one comprehensive, generic margin of 7 mm, the CovP-strategy includes patient-specific organ movement information, which leads to a more specific, but larger PTV. The negative consequences of the larger PTV are in turn compensated by a more relaxed CovP dose prescription which allows controlled underdosages in locations with a low probability of CTV presence. Direct comparisons of cPTV-DVHs are naturally biased in favor of the cPTV/image guided RT (IGRT) strategy for two reasons: the PTV is smaller, and the dose is optimized with less regard to the normal tissues. In order to compare like with like, we based our analysis on the cPTV-based single-geometry-instance PTV. Thus, the analysis answers the question of how much the PTV dose would suffer from organ movement for a dose distribution obtained via conventional prescription vs. a dose distribution obtained via CovP-dose prescription including patient-specific motion information. The CovP-concept showed higher robustness in target volume coverage without target miss until 70 Gy, compared to a conventional PTV-based IMRT. In addition, OAR-sparing at harmful radiation doses (≥70Gy rectum, ≥74Gy bladder) was significantly better with the CovP-concept.
In general, the coverage probability concept was developed as a pre-adaptive concept based on several CTs (Citation11,Citation12). The assumed advantage of the CovP-concept is that individual positional displacements, deformations of the prostate and different filling conditions of bladder and bowel were probabilistically considered in the treatment plan. Both concepts (CovP and conventional PTV-based IMRT) were used with the same safety margins of 7 mm. The difference of both treatment concepts was demonstrated for the coverage of this safety margin. The conventional static PTV-margin and the probabilistic consideration of presence of OARs and target volumes in the CovP-concept resulted in superior OAR sparing, avoiding of target miss and comparable 95% isodoses for the target volume with the CovP-concept.
The common way to handle uncompensated treatment uncertainties is by the addition of margins and the requirement of a homogeneous dose prescription for the resulting PTV. The combination of these two elements leads to the problem that overlap regions with OARs are created that require, according to the rules, almost the full target dose. In general, the more uncertainties are compensated by margins, the more serious the overlap problem becomes. The most widely practiced amelioration strategy is real-time image-guidance and adaptation of the treatment, such as setup correction, plan adaptation or real-time plan selection.
Another way of uncertainty compensation is offered by the CovP-concept, which essentially maintains the margins of the PTV concept, but replaces the rigid dose prescription by one that is adapted to the spatial probability of organ presence. This alleviates the paradoxical situation of overlaps between the PTV and OARs to some extent, but also requires some a-priori information about the treatment uncertainties, like all schemes of robust dose optimization (Supplemental Table 1, (Citation14)).
The plan quality was evaluated by comparing the DVH-parameters of a virtual “conventional” IMRT plan. We chose to investigate doses to PTV (CovP-ITV) rather than CTV dose for this novel RT concept firstly since PTV is supposed to be irradiated with the prescribed dose and this represents clinical routine. Secondly, this concept also factors in OAR presence in PTV (and not only vice versa) and therefore PTV dose seems of higher interest. The comparison of the DVH-parameters of the CovP-ITV with the cPTV showed that the CovP-plan covered the target volume of day 1-3 better up to V70 without target miss. The cPTV plan reached relevant missed volumes at 60-70 Gy (V70 = 97.2%). Per additional Gy, biochemical control is increased by 1.78% for intermediate risk patients (Citation15). However, dose-escalation according to the prescribed dose of 78 Gy was superior in the cPTV-plan going at the expense of both OARs (rectum and bladder) in the high-dose range (V70-V78), especially on geometry instances 2 and 3. However, the achieved doses in the middle dose range on the rectum were slightly higher with the CovP-concept. Yet, all of Quantec’s constraints were met by both plans (Citation16). Therefore, a negative effect of this dose difference at V40-60 in terms of increased side effects might not be expected. Potentially, further plan optimization in the CovP-concept would have been possible, but this was not pursued because of the achievement of the set goal parameters (DVH-constraints). In contrast, the dose increase at V70+ in the PTV-based plan for bladder and rectum (days 2-3) is less acceptable, since currently used more restrictive thresholds are exceeded (bladder/rectal V75 < 5%, rectum V70 < 15%) (XXX). The therapeutic ratio of the CovP-concept seems to be favorable regarding target coverage and high-dose OAR-sparing compared to the conventional PTV-based plan.
From a cost-benefit point of view, the CovP-concept is significantly more complex, because instead of one CT, three CTs are performed and contoured. Financial burden and processing time are higher. For the patient, radiation dose is increased by two additional CTs. This is, from our point of view, justified by the better, more robust dose-distribution, which itself reduces unnecessarily placed radiotoxicity. If target volume and OAR-movements are minimal over three days of consecutive planning CTs, then the benefit of the CovP-concept might be regarded as negligible. However, in this case daily image verification during the course of RT can be reduced to one or two cone-beam CTs (CBCT) per week, also reducing radiation exposure to the patient by CovP-concept.
Although state of the art IGRT is recommended in current guidelines (Citation4), the potential advantage of the robust CovP-concept is that daily CBCT would not be necessary to compensate for position errors, leading to less radiation exposure from CBCTs and accelerating the daily workflow (Citation17). Otherwise, the benefit of CovP is that individual deformation of target- and OAR volumes is considered in the treatment plan which cannot be balanced by daily correction of isocenter shifts, i.e., image guidance.
In general, deviations in the first 5 min are in the range of 5 mm - 10 mm (Citation18). Therefore, an additional advantage might be increased compensation for individual intra-fractional shifts and hence target miss prevention. This benefit - implementation of deformation instead of organ shifts - remains also if IGRT is performed. Evidence for this line of reasoning is the current implementation of this concept in a cervical carcinoma study (Citation19). A potential bias is that the three CTs consider only a portion of the anatomical changes during 39 fractions of RT. However, long-term outcome data for the 27 patients in this prospective study is also excellent (Citation13), hinting that no major target miss occurred. In addition, the applicability of 3 CTs to sufficiently cover the prostate was shown by evaluations using more than 3 CTs (Citation11,Citation20).
Modern adaptive procedures with online-plan-generation and -adaptation can further optimize the therapy and reduce margins (MR-LINAC) (Citation21). Yet adaptive treatment requires online presence of a radiation oncologist and a physicist and much additional time for both for daily re-planning, limiting its general application (Citation22) in contrast to CovP. Other adaptive concepts such as “plan of the day” also need a daily assistance of a radiation oncologist to decide which plan fits best (Citation23) and also depend on input of several geometry instances. But the issue of intrafractional variability is still not solved. Here, an adaptive image-guided CovP-concept also implementing fractional variability at the start of IGRT could further reduce geometrical uncertainties during treatment.
This study has the following limitations: The sample size of n = 27 is reasonable for a monocentric prospective study, yet further evaluation of the CovP-concept with a larger patient collective is needed. Also, the comparison of CovP-plan parameters with a conventional IMRT plan based on one CT could only be performed in silico and no intraindividual patient data is available. Further comparisons with more advanced radiotherapy techniques such as IGRT or online adaptive RT can only be performed indirectly through comparisons with the PTV-based IMRT plan.
Conclusion
Patient-specific information about organ movement improves PTV definition by elimination of systematic organ deformation uncertainties. The CovP-concept implements a robust optimization strategy, which showed higher target volume coverage without target miss until 70 Gy, compared to a conventional PTV-based IMRT-plan. In addition, negative consequences of inflated PTV can be ameliorated by more relaxed, CovP-based dose prescription.
| Abbreviations | ||
| CovP | = | coverage probability |
| CT | = | computed tomography |
| CBCT | = | cone beam CT |
| CTC | = | common toxicity criteria |
| CTV | = | clinical target volume |
| DVH | = | dose–volume histogram |
| EUD | = | equivalent uniform dose |
| GI | = | gastrointestinal |
| IGRT | = | image-guided radiation therapy |
| IMRT | = | intensity-modulated radiotherapy |
| ITV | = | internal target volume |
| OAR | = | organ at risks |
| PC | = | prostate cancer |
| PRV | = | planning organ at risk volume |
| PTV | = | planning target volume |
| RT | = | radiotherapy |
| RTOG | = | Radiation Therapy Oncology Group |
| VX | = | volume receiving at least X Gray |
Supplemental Material
Download MS Word (30 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Yu T, Zhang Q, Zheng T, et al. The effectiveness of intensity modulated radiation therapy versus three-dimensional radiation therapy in prostate cancer: a meta-analysis of the literatures. PLoS One. 2016;11(5):e0154499.
- Bauman G, Rumble RB, Chen J, et al. Intensity-modulated radiotherapy in the treatment of prostate cancer. Clin Oncol. 2012;24(7):461–473.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Prostate Cancer Version 1.2020. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020. Available from: www.nccn.org
- Leitlinienprogramm Onkologie der AWMW DKeVu, e.V DK. Interdisziplinäre leitlinie der qualität s3 zur früherkennung, diagnose und therapie der verschiedenen stadien des prostatakarzinoms. German S3-Guideline. Langversion 5.0. 2018;1–394. Available from: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Prostata_5_0/LL_Prostata_Langversion_5.0.pdf
- Thorwarth D, Notohamiprodjo M, Zips D, et al. Personalized precision radiotherapy by integration of multi-parametric functional and biological imaging in prostate cancer: a feasibility study. Z Med Phys. 2017;27(1):21–30.
- Engels B, Soete G, Gevaert T, et al. Impact of planning target volume margins and rectal distention on biochemical failure in image-guided radiotherapy of prostate cancer. Radiother Oncol. 2014;111(1):106–109.
- Storey MR, Pollack A, Zagars G, et al. Complications from radiotherapy dose escalation in prostate cancer: preliminary results of a randomized trial. Int J Radiat Oncol Biol Phys. 2000;48(3):635–642.
- Michalski JM, Bae K, Roach M, et al. Long-term toxicity following 3d conformal radiation therapy for prostate cancer from the rtog 9406 phase i/ii dose escalation study. Int J Radiat Oncol Biol Phys. 2010;76(1):14–22.
- Eckert F, Alloussi S, Paulsen F, et al. Prospective evaluation of a hydrogel spacer for rectal separation in dose-escalated intensity-modulated radiotherapy for clinically localized prostate cancer. BMC Cancer. 2013;13(1):27.
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 chhip trial. Lancet Oncol. 2016;17(8):1047–1060.
- Baum C, Alber M, Birkner M, et al. Robust treatment planning for intensity modulated radiotherapy of prostate cancer based on coverage probabilities. Radiother Oncol. 2006;78(1):27–35.
- Baum C, Birkner M, Alber M, et al. Dosimetric consequences of the application of off-line setup error correction protocols and a hull-volume definition strategy for intensity modulated radiotherapy of prostate cancer. Radiother Oncol. 2005;76:35–42.
- Wegener D, Berger B, Outaggarts Z, et al. Probabilistic planning concept instead of target volume margins - prospective evaluation. Radiother Oncol. 2018;127:S864–S865.
- Unkelbach J, Alber M, Bangert M, et al. Robust radiotherapy planning. Phys Med Biol. 2018;63(22):22TR02–22TR02.
- Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74(5):1405–1418.
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. *International Journal of Radiation Oncology*Biology*Physics. 2010;76(3):S10–S19.
- Tøndel H, Lund J-Å, Lydersen S, et al. Radiotherapy for prostate cancer – does daily image guidance with tighter margins improve patient reported outcomes compared to weekly orthogonal verified irradiation? Results from a randomized controlled trial. Radiother Oncol. 2018;126(2):229–235.
- Ballhausen H, Li M, Hegemann NS, et al. Intra-fraction motion of the prostate is a random walk. Phys Med Biol. 2015;60(2):549–563.
- Ramlov A, Assenholt MS, Jensen MF, et al. Clinical implementation of coverage probability planning for nodal boosting in locally advanced cervical cancer. Radiother Oncol. 2017;123(1):158–163.
- Yan D, Lockman D, Brabbins D, et al. An off-line strategy for constructing a patient-specific planning target volume in adaptive treatment process for prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48(1):289–302.
- Wegener D, Zips D, Thorwarth D, et al. Precision of t2 tse mri-ct-image fusions based on gold fiducials and repetitive t2 tse mri-mri-fusions for adaptive igrt of prostate cancer by using phantom and patient data. Acta Oncol. 2019;58(1):88–94.
- McPartlin AJ, Li XA, Kershaw LE, et al. Mri-guided prostate adaptive radiotherapy – a systematic review. Radiother Oncol. 2016;119(3):371–380.
- Burridge N, Amer A, Marchant T, et al. Online adaptive radiotherapy of the bladder: small bowel irradiated-volume reduction. Inter J Radiation Oncol Biol Phys. 2006;66(3):892–897.