 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose: Ruthenium-106 (Ru-106) brachytherapy is a common eye-preserving treatment for choroidal melanomas. However, a dose-response model describing the relationship between the actual delivered tumour dose and tumour control has, to the best of our knowledge, not previously been quantified for Ru-106 brachytherapy; we aimed to rectify this.
Material and methods: We considered consecutive patients with primary choroidal melanomas, treated with Ru-106 brachytherapy (2005–2014). Dosimetric plans were retrospectively recreated using 3D image-guided planning software. Pre-treatment fundus photographies were used to contour the tumour; post-treatment photographies to determine the accurate plaque position. Patient and tumour characteristics, treatment details, dose volume histograms, and clinical outcomes were extracted. Median follow-up was 5.0 years. The relationship between tumour dose and risk of local recurrence was examined using multivariate Cox regression modelling, with minimum physical tumour dose (D99%) as primary dose metric.
Results: We included 227 patients with median tumour height and largest base dimension of 4 mm (range 1–12, IQR 3–6) and 11 mm (range 4–23, IQR 9–13). The estimated 3 year local control was 82% (95% CI 77–88). Median D99% was 105 Gy (range 6–783, IQR 65–138); this was the most significant factor associated with recurrence (p < .0001), although tumour height, combined TTT and Ru-106 brachytherapy, and sex were also significant. The hazard ratio (HR) for a 10 Gy increase in D99% was 0.87 (95% CI 0.82–0.93). Using biological effective dose in the model resulted in no substantial difference in dose dependence estimates. Robustness cheques with D1–99% showed D99% to be the most significant dose metric for local recurrence.
Conclusion: The minimum tumour dose correlated strongly with risk of tumour recurrence, with 100 Gy needed to ensure at least 84% local control at 3 years.
Introduction
Brachytherapy is commonly used as eye-preserving treatment for choroidal melanoma; with no reported difference in survival between brachytherapy compared to enucleation for medium-sized tumours [Citation1]. Different types of isotopes are in use, but Ruthenium-106 (Ru-106) is the most regularly used in Europe [Citation2]. Radiotherapy of the eye aim at attaining local tumour control while sparing the healthy adjacent structures. Based on long clinical practice, a minimal apical dose of 80–100 Gy has generally been accepted as an acceptable prescription dose to achieve this goal [Citation2–4], although compromise on tumour coverage is sometimes accepted to spare organs at risk. A dose-response model describing the relationship between the actual delivered tumour dose and tumour control has, however, not previously been quantified for Ru-106 brachytherapy. The development of tumour control probability (TCP)-models could aid the understanding of this relationship and support optimisation of future treatments. We hypothesised that for uveal melanoma patients, the minimum dose delivered to the tumour, assessed using full 3D dose calculation, could be related to risk of tumour recurrence, and examined this in a large retrospective cohort.
Material and methods
Patients
Consecutive patients treated at our institution with Ru-106 brachytherapy for primary choroidal melanoma between January 2005 and December 2014 were included. Patients were generally referred to brachytherapy if they had locally confined disease and the tumour dimensions were within the limits treatable with Ru-106 ophthalmic plaques (5 mm in height). Tumours with larger height than 5 mm (n = 73) were treated if there was a strong patient-preference for eye-preserving treatment, following a thorough discussion of treatment options. Since enucleation was the only alternative treatment available at our institution, especially monocular patients did in some cases prefer Ru-106 brachytherapy.
All patients were followed up regularly; every third month for the first year, every sixth month the second year, annually up to 5 years and then at 7, 10 and 12 years after primary treatment. Patients who developed distant metastases continued the follow-up schedule for as long as possible.
Slit lamp examinations, fundus photographies and ultrasound B-scans were performed on all patients both before treatment and continuously during follow-up. Image material was thus available for the vast majority of patients. Tumour control was defined as complete tumour regression or regression to a stable condition without any signs of tumour growth. Recurrence was defined as an increase in either tumour height or basal diameter (examined using ultrasound B-scans) compared to the previous assessments on two consecutive measurements.
Patient characteristics, including age, sex, and incident eye, were extracted retrospectively along with details on each specific treatment session, including tumour characteristics and dose data.
Treatment planning and delivery
Treatments were performed in an operating theatre with the patient in general anaesthesia. The plaque was sutured to the sclera adjacent to the tumour and removed when a prescribed dose of 100 Gy to the tumour apex had been delivered. A 2 mm margin was generally preferred but an eccentrically located plaque was used in some cases, especially for tumours in close proximity to the macula and/or the optic disc. Extraocular muscles were detached when relevant. The treatment time was calculated using an in-house developed spreadsheet using only the activity of the plaque at insertion time and the dose depth (tumour height at the apex and scleral thickness). The dose depth was measured using ultrasound B-scans during plaque insertion utilising the mirror image artefact [Citation5]. Ultrasound was additionally used intraoperative (directly after plaque placement) and postoperative to ensure correct plaque positioning and to detect if bleeding behind the plaque was present. Four different Ru-106 plaque types were available (CCA, CCB, CCC, and COB, all from Eckert and Ziegler BEBIG, GmbH, Berlin Germany) and used according to tumour size and/or location within the globe. The plaques were renewed every 9–12 months with both a newly produced set and an older set kept in the department, to be used according to specific tumour characteristics i.e., tumour height. The initial activities were measured by the manufacturer and varied from plaque to plaque. Before 2008, Ru-106 brachytherapy was supplemented by transpupillary thermotherapy (TTT) if indicated by minimal or absent tumour shrinkage, however with no signs of recurrence. During the 10 year period, three experienced ophthalmologists performed the surgeries.
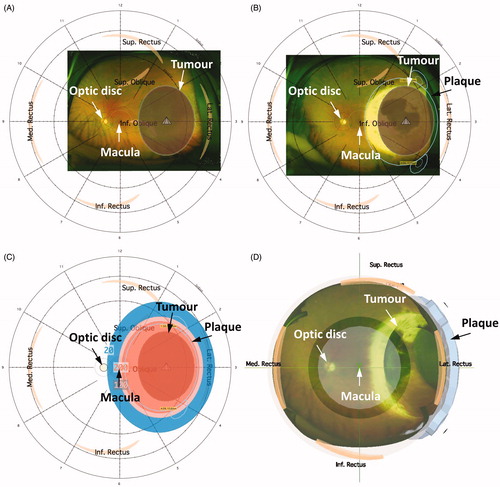
Treatment dose distributions were retrospectively recreated () using the 3D image-guided planning software Plaque Simulator (version 6.5.9, EyePhysics, LLC, Los Alamitos, CA, USA). This system calculates the dose on a standard eye (anterior-posterior diameter of 26.2 mm and equatorial diameter of 24.0 mm) using calculation analogous to the American Association of Physicists in Medicine Task Group No. 43 brachytherapy formalism (AAPM TG43) [Citation6,Citation7]. The images were registered on the standard eye using landmarks (macula and optic disc). Pre-treatment fundus photographies enabled contouring of the tumour base whereas the tumour height and scleral thickness were extracted from the ultrasound measures enabling a 3D recreation of the tumour volume. If the pre-treatment fundus photography was unavailable, the tumour position was determined from the patient notes. In most cases, a radiation scar could be identified on the retina and visualised on the post-treatment fundus photographies which facilitated direct determination of accurate plaque position. If a clear radiation scar had not developed (or if post-treatment fundus photograhies were not available), the description of plaque position from the surgical note was used. The exact treatment time was extracted from the patient records allowing 3D dose distributions to be recreated.
Figure 1. 3D image-guided planning using Plaque Simulator. (A) Recreated tumour position and relative distances to the macula and the optic disc on pre-treatment fundus photography. (B) Retrospectively recreated plaque position based on radiation scar on post-treatment fundus photography. (C) 3D dose distributions were recreated based on the exact treatment time extracted for each patient. The 200 Gy, 100 Gy, and 20 Gy isodose lines are shown. (D) 3D illustration of the recreated treatment plan showing plaque position, the tumour, macula, and optic disc. An anterior view was chosen for illustration purposes.
Dose volume histograms for the tumour, the macula, and the optic disc were extracted from each plan. In addition to the physical doses (D), biologically effective doses (BED) were estimated to account for dose-rate effects, using a well-established model for continuous low dose rate brachytherapy (CLDR) from Fowler et al. [Citation8] (EquationEquation (1)(1)
(1) )
(1)
(1)
Dose D, treatment time t, source half time of T1/2 = 1.5 h (corresponding to μ ), and the tissue specific factor α/βtumour = 11.5 Gy [Citation9].
Data analysis and modelling
The overall local recurrence rate was assessed using Kaplan-Meier estimates. Additional Kaplan-Meier estimates stratifying for specific clinical factors (D99%, optic disc-tumour distance, tumour height, and stage (as defined by the American Joint Committee on Cancer (AJCC) [Citation10]) were produced for descriptive purposes.
The relationship between minimum physical dose to the tumour (dose to 99% of the tumour volume, D99%) and risk of recurrence was examined with multivariate Cox regression modelling, taking clinical factors into account. Before the analysis, a visual correlation check was performed to avoid problems with collinearity. When two variables correlated, we kept the variable judged most clinically relevant. See Figure 4 in the Supplementary material for more details on the correlation analysis. The exception was tumour height and tumour AJCC staging which correlated closely, but which are of independent clinical interest. Consequently, two analyses were performed; one in which tumour dose, combined Ru-106 brachytherapy and TTT treatment (see below), optic disc-tumour distance, tumour height, patient age at treatment, incident eye, and sex were included; and one in which tumour height was replaced by staging but all other factors retained. The full models were reduced by backward selection until only significant (p < .05) covariates remained.
A small subset of patients received Ru-106 brachytherapy in combination with TTT as primary treatment. These were handled similar to the remaining cohort, but accounting for use of TTT as an explanatory factor in the statistical analysis. If TTT was used after the primary treatment (i.e., during follow-up), patients were censored at that time.
The time variable used in the analysis was defined as the time from start of the Ru-106 treatment until recurrence, TTT in follow-up, death, or study cut-off date (June 2018). The inverse Kaplan-Meier estimate was used to determine the median potential follow-up time [Citation11].
Model robustness was assessed by considering alternative dose metrics (D1%-99%), BED (BED1%-99%), and by taking the competing risks of death and TTT (after primary treatment) into account in cumulative incidence modelling. The Aalen-Johansen estimator was used for cumulative incidence, while Fine and Gray’s model was used for regression analysis. Model calibration was assessed by the correlation between observed and predicted 3 year tumour control.
To explore whether risk factors for recurrence (including the dose-dependence, considering the full dose range) were different for different types of tumour regrowth, we fitted competing risk regression models for both marginal and central tumour recurrence, with death and TTT in follow-up as additional competing risks. Fine and Gray’s model was used for regression analysis.
Dose-response of tumour control was visualised by plotting tumour control probability at fixed time points (3 and 7 years), as predicted from Cox regression models, as a function of dose, with all other model variables kept constant. Additionally, the impact of tumour height on TCP was demonstrated by varying the tumour heights using the mean from each AJCC staging group (I-III) [Citation10].
Calibration plots were made for the reduced Cox models (with D99% and BED99%, respectively) to visualise correspondence between predicted and observed 3 year local tumour control rates. Intervals with 40 patients in each were used, and resampling (500 times) was used for confidence intervals (Figure 10 in the Supplementary material).
We checked the Cox model assumption of proportionality over time by examining model residuals for all covariates and testing for time dependence.
All analyses were conducted with R (version 3.6.1) in R Studio (version 1.0.153).
Results
Two hundred twenty-seven choroidal melanoma patients were treated in a 10 year time period. Of those, 226 were eligible for analysis. Six of these had limited follow-up (see the Supplementary material for details). All other patients were followed until local recurrence, death, or study cut-off. Patient, tumour, treatment and recurrence characteristics are listed in .
Table 1. Patient-, tumour-, and treatment characteristics.
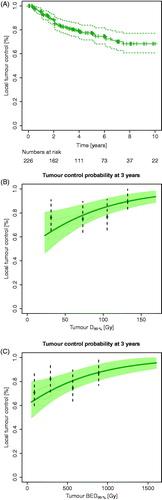
The median follow-up was 5.0 years. Fifty (22%) experienced local recurrence, and 79 died (49 due to uveal melanoma metastases, 14 due to other cancers, and 16 due to other causes). The estimated 3 year local control was 82% (95% CI: 77–88) ().
Figure 2. (A) A Kaplan-Meier curve for local control for the entire patient population. Dotted lines: 95% confidence intervals; crosses: censored patients. (B) Tumour control probability (TCP) at 3 years with 95% confidence interval using D99%. The TCP curve adjusted for tumour height of 3.9 mm (median height of cohort), no combined TTT and Ru-106 treatment (most common in the cohort), and male sex (most frequent in the cohort). (C) TCP curve at 3 years using BED99%. The TCP curve used no combined TTT and Ru-106 treatment (most common in the cohort) and male sex (most frequent in the cohort). Both TCP curves (B and C) were based on Cox proportional hazard regression, and the data points represent Kaplan-Meier estimates at 3 years after stratifying into four dose groups (for illustration purpose only).
The results from the additional univariate Kaplan-Meier analyses with patients divided by clinical variables are illustrated in Figure 5 in the Supplementary material.
lists the hazard ratios (HR) and 95% confidence intervals (CI) for D99% and all other covariates from the full multivariate Cox model and the corresponding reduced model. Note that the HR for D99% is reported for a 10 Gy increase in D99%.
Table 2. Cox proportional hazards.
The reduced model had D99% as the main significant parameter, with HR for a 10 Gy increase in D99% of 0.87 (95% CI 0.82–0.93). Besides D99%, tumour height, combined TTT and Ru-106 brachytherapy and sex were significantly related to local tumour control.
The model was robust for use of BED rather than physical dose (Table 4 in the Supplementary material), however, the effect of tumour height proved to be less robust and thus not significant in the model. The dose-responses using D99% and BED99% are illustrated in ), using tumour control at 3 years for visualisation. The model adjusts for tumour height, combined TTT and Ru-106 treatment, and sex. As seen in , for an average patient with a median tumour height (3.9 mm), the estimated tumour control at 3 years increased from 81% with a minimum tumour dose of 85 Gy to 89% for 130 Gy.
A separate model was optimised with AJCC stage as an alternative to tumour height but with all other factors retained (Table 3 in the Supplementary material). The model was robust, and all variables remained significant.
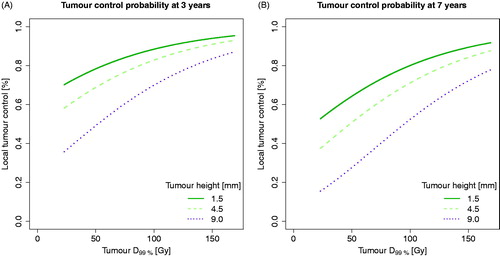
illustrate dose-response models at 3 and 7 years for D99% divided into three tumour heights (based on mean values of each tumour staging group; 1.5 mm, 4.5 mm, and 9.0 mm). Increase in tumour height correlated with worse local control. See Figure 6 in the Supplementary material for the corresponding TCP models divided into staging groups.
Figure 3. Tumour control probability (TCP) curves taking dose and tumour height into account (tumour heights based on mean values of each tumour staging group). (A) TCP for D99% at 3 years. (B) TCP for D99% at 7 years. TCP curves were based on Cox proportional hazard regression, using no combined TTT and Ru-106 treatment (most common in the cohort) and male sex (the most frequent sex in the cohort).
None of the alternative dose metrics (D1%–98%) were found to correlate better to local tumour control than to D99%, based on dose metric p-values. Similar results were found for BED. The p-values for the full range of dose metrics in the reduced Cox model are plotted in Figure 7 in the Supplementary material for physical doses and BED.
Calibration plots demonstrated good agreement between predicted and observed local control rates for both reduced models (Figure 10 in the Supplementary material).
Competing risk analysis showed cumulative incidences at 3 years for recurrence, death, and TTT of 15% (95% CI: 11–20), 14% (95% CI: 9–18), and 6% (95% CI: 3–9), see Figure 8 in the Supplementary material. Accounting for death and TTT, D99% remained the most significant factor for recurrence with HR for a 10 Gy increase of 0.87 (0.82–0.92, p < 10−4). Additionally, combined TTT and Ru-106 brachytherapy and sex remained significant explanatory variables, while tumour height showed borderline significance. See Table 5 in the Supplementary material for results from the cumulative incidence model. Modelling marginal and central tumour recurrence separately (and considering the other type of recurrence a competing risk), we found a clear dependence of dose and tumour height for central recurrences, but not for marginal recurrences. See Figure 9 and Tables 6–7 in the Supplementary material for details.
None of the underlying Cox proportionality assumptions were violated.
Discussion
We report results from a complete dataset of consecutive patients treated with Ru-106 brachytherapy in the period 2005–2014. The follow-up is consistent and only a single patient was lost to follow-up before any routine control visits.
Dose-response relationships for uveal melanomas treated with Ru-106 brachytherapy have, to the best of our knowledge, not previously been reported in the literature, making the results of this work highly relevant for current clinical practice and for treatment guidelines. The estimated minimum dose to the tumour proved to correlate strongly with risk of tumour recurrence, with a physical dose of 100 Gy needed to ensure at least 84% local control at 3 years. Controversy exists in the literature – and in clinical practise – around the optimal apex prescription dose, with reported values ranging from 85 to 130 Gy [Citation12–16]. Based on our model, the corresponding estimated 3 year tumour control rates for these apical doses would be 81% and 89%, respectively. In addition to an apical prescription dose of 130 Gy, Stöckel et al. [Citation16] use a restriction of at least 700 Gy to the base of the tumour to introduce an increased treatment margin, accounting for possible uncertainties in plaque placements and thus dose distributions to the tumour. This larger prescription dose might thus be considered for future treatments, but it should be carefully compared to the risk of any visual acuity decreasing side-effects.
Treatment plans were recreated using 3D image-guided software enabling accurate plaque position to be determined from follow-up fundus photographies. The analysis was consequently based on the actual delivered 3D dose distributions and not point doses. As such, the D99% directly reflects any geographic miss (the minimum dose to the tumour will be low); while for tumours with full dose coverage, it might correlate closely to the prescription dose.
In our primary analysis, we did not distinguish between marginal and central recurrences. For the purpose of treatment plan optimisation and individualisation, we are primarily interested in the total recurrence risk estimate as a function of minimum dose. In order to further understand recurrence patterns (and possible guide optimal choice of therapy), it may additionally be of interest to examine whether the two types of recurrence are one or different phenotypes. Our secondary analyses, separating the two types of recurrence and finding only dose dependence for central recurrences, provide a tentative indication that this might be the case.
Local control rates vary considerably throughout literature and range from a 5 year probability of 59% [Citation17] to 97.9% [Citation12]. Damato et al. who observed nine local recurrences (out of 458 patients), report on a highly selected cohort (median tumour height 3.2 mm, range 0.7–7.0 mm), for whom Ru-106 brachytherapy was the most convenient treatment modality. In contrast to our results, they found that dose was not a significant risk factor for recurrence and report largest base dimension as the only significant risk factor, although with no multivariate analysis. Marconi et al. [Citation14] reported local tumour control of 93.6%, with increased risk of local recurrence with lower apical dose, which agrees with our findings. Isager et al. [Citation18] reported 5 year local tumour control of 73%, and found anterior location, largest base dimension and tumour height as significant risk factors for local tumour recurrence, which is partly in line with our findings.
The dose-response relationship observed in the current study was somewhat shallow, and we did observe recurrent events, even for 15 cases in which the tumour was calculated to have received >100 Gy. A sub analysis showed that the majority of these were either large tumours or near the optic disc, but the remaining 5 cases could not be explained by such characteristics. This number is comparable to the selected cohort by Damato et al. [Citation12]. It has previously been suggested that specific gene defects might result in radiation resistance. This phenomenon is currently being studied further [Citation19]. Our results could indicate some extent of radiation resistance, but we did not have sufficient genetic information to investigate this.
Sex remained a significant explanatory variable for local recurrence in the robustness analyses and when accounting for competing risks. Other studies have found male sex to have earlier and more frequent metastases in the first decade after the diagnosis of uveal melanomas [Citation20], but we did not have a robust clinical explanation for this finding in regard to local recurrence of uveal melanoma.
We included patients with tumour heights up to 12 mm, larger than the traditionally recommended 5 mm [Citation21]. Kaiserman et al. [Citation22] concluded that brachytherapy provided acceptable results for some tumours with heights of more than 8 mm, and they reported a 5 year local tumour control rate of 76%. In our work, however, tumour height proved to have a significant negative influence on the local control probability, even when accounting for minimum tumour dose. This finding was supported by Brualla et al. [Citation23] who suggested to avoid Ru-106 brachytherapy of tumours more than 5 mm of height due to very large doses to the sclera and the healthy structures at risk.
Our results indicate that different treatment modalities should potentially be considered for large tumours. It is, however, not obvious which treatment modality that should be used: Both Iodine-125 and proton therapy have shown acceptable results for larger tumours in regard to local control rates [Citation24–26]. The optimal choice between the two (taking into account logistical challenges as well) for each individual patient may have to be the subject of further studies.
Yarovoy et al. [Citation27] found improved local tumour control for patients treated with brachytherapy combined with TTT compared to brachytherapy alone. When we adjusted for stage in the reduced multivariate Cox analysis (Table 3 in the Supplementary material), we found combined treatments to have a significant negative impact on local tumour control. During the time period of this study, TTT was delivered as supplementary treatment in cases with expected inferior response to Ru-106 treatments; e.g., large tumours and tumours in close proximity to the optic disc. It was furthermore used in cases with poor tumour regression or re-growth; for the latter indicating biologically more aggressive and/or radiation resistant tumours [Citation19,Citation28]. Since effect of TTT on tumour control is unclear, and likely small, this use is unlikely to have biased our radiation dose-response estimate.
Model robustness was assessed using BED which did not change the overall results in the current study (), and we observed a similar correlation between BED99% and recurrence to that found when using physical dose. Combined with an acceptable correlation between predicted and observed 3 year local control, we believe that the established model is reliable. It is, however, important to emphasise the limitations in the data underlying our models. Importantly, we developed the models using dose estimates from recreated 3D dose distributions. These were based on (image-guided) assumptions regarding plaque positioning and activity as provided by the plaque manufacturer’s empirical measurements. It should, additionally, be kept in mind that the treatment plans were made using a standard eye size. Since all eyes are not of equal sizes, this is a limitation to the recreated treatment plans. BED estimates are limited by the parameters involved (T1/2 and α/β). Values used are the best currently available in the literature, but they are largely based on in vivo data.
There is no established standard for dose reporting for ocular brachytherapy [Citation29]. Reports from the GEC-ESTRO committee and The American Association of Physicists in Medicine (AAPM) have provided recommendations on dose reporting in various other tumour sites traditionally treated with brachytherapy, including gynaecological and prostate cancer [Citation30–32]. Generally, reporting of minimum or near-minimum dose to the tumour volume (e.g., D100%, D99%, D98% and D90%) is recommended. This is in line with the guidelines for external beam radiotherapy, described in Report 83 from the International Commission on Radiation Units and Measurements (ICRU) [Citation33]. Heileman et al. [Citation34] use D98% in their study of treatment optimisation of Ru-106 brachytherapy. We considered various alternative dose metrics as part of our secondary robustness analyses and tested the significance of the full range (D1%–D99%). We found D99% to correlate strongly with outcome, with no additional advantage of using other dose metrics.
While tumour control is important, healthy tissue toxicity should also be considered and assessed when deciding the optimal treatment for each individual patient. Prognostic factors such as distance and dose to structures at risk, diabetes, and tumour volume have been evaluated for radiation-induced side effects after Ru-106 treatments for choroidal melanomas [Citation35,Citation36]. A normal tissue complication probability analysis for the present cohort has recently been published by our group [Citation37].
Our TCP analysis allows the ophthalmologist to quantify the likely change in TCP arising from a suboptimal plaque position (e.g., due to adjacent anatomical structures making optimal plaque positioning difficult) or altered treatment time (e.g., arising from the surgical theatre being unavailable). It would be highly relevant and necessary to validate the model in an external dataset. Until then, these results represent the only available dose-response relationship for the probability of local tumour control for choroidal melanoma patients treated with Ru-106 brachytherapy.
Conclusions
We have established tumour dose-response relationships for uveal melanoma patients treated with Ru-106 brachytherapy. Minimum dose delivered to the entire tumour volume correlated strongly with the risk of tumour recurrence, with 100 Gy needed to ensure at least 84% local control at 3 years.
Supplemental Material
Download PDF (754.6 KB)Acknowledgments
This work is supported by research grants from Synoptik-Foundation, the Danish Eye Research Foundation (Øjenfonden), the Danish Cancer Research Foundation (Dansk Kræftforsknings Fond), Aase and Ejnar Danielsens Foundation, and Arvid Nilssons Foundation. Ane Appelt is supported by Yorkshire Cancer Research Academic Fellowship funding (grant L389AA). None of the funders had any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119(7):969–982.
- Simpson ER, Gallie B, Laperrierre N, et al. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13(1):1–14.
- Damato B, Patel I, Campbell IR, et al. Visual acuity after Ruthenium106 brachytherapy of choroidal melanomas. Int J Radiat Oncol. 2005;63(2):392–400.
- Giblin ME, Shields JA, Augsburger JJ, et al. Episcleral plaque radiotherapy for uveal melanoma. Aust N Z J Ophthalmol. 1989;17(2):153–156.
- Espensen CA, Jensen PK, Fog LS, et al. Ultrasonic mirror image from ruthenium plaque facilitates calculation of uveal melanoma treatment dose. Br J Ophthalmol. 2017;101(9):1206–1210.
- Astrahan M. a. A patch source model for treatment planning of ruthenium ophthalmic applicators. Med Phys. 2003;30(6):1219–1228.
- Rivard MJ, Coursey BM, DeWerd L., et al. Update of AAPM Task Group No. 43 Report: a revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004;31(3):633–674.
- Fowler JF. 21 Years of biologically effective dose. BJR. 2010;83(991):554–568.
- Gagne NL, Leonard KL, Huber KE, et al. BEDVH—a method for evaluating biologically effective dose volume histograms: application to eye plaque brachytherapy implants. Med Phys. 2012;39(2):976–983.
- Kujala E, Damato B, Coupland SE, et al. Staging of ciliary body and choroidal melanomas based on anatomic extent. J Clin Oncol. 2013;31(22):2825–2831.
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346.
- Damato B, Patel I, Campbell IR, et al. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol. 2005;63(2):385–391.
- Browne AW, Dandapani SV, Jennelle R, et al. Outcomes of medium choroidal melanomas treated with ruthenium brachytherapy guided by three-dimensional pretreatment modeling. Brachytherapy. 2015;14(5):718–725.
- Marconi DG, De Castro DG, Rebouças LM, et al. Tumor control, eye preservation, and visual outcomes of ruthenium plaque brachytherapy for choroidal melanoma. Brachytherapy. 2013;12(3):235–239.
- Bergman L, Nilsson B, Lundell G, et al. Ruthenium brachytherapy for uveal melanoma, 1979–2003: survival and functional outcomes in the Swedish population. Ophthalmology. 2005;112(5):834–840.
- Stöckel E, Eichmann M, Flühs D, et al. Dose distributions and treatment margins in ocular brachytherapy with 106Ru eye plaques. Ocul Oncol Pathol. 2018;4(2):122–128.
- Summanen P, Immonen I, Heikkonen J, et al. Survival of patients and metastatic and local recurrent tumor growth in malignant melanoma of the uvea after ruthenium plaque radiotherapy. Ophthalmic Surg. 1993;24(2):82–90.
- Isager P, Ehlers N, Urbak SF, et al. Visual outcome, local tumour control, and eye preservation after106Ru/Rh brachytherapy for choroidal melanoma. Acta Oncol (Madr). 2006;45(3):285–293.
- Dogrusöz M. Genetic Prognostication in Uveal Melanoma. Netherland: University of Leiden, 2018.
- Zloto O, Pe'er J, Frenkel S. Gender differences in clinical presentation and prognosis of uveal melanoma. Invest Ophthalmol Vis Sci. 2013;54(1):652–656.
- Eckert and Ziegler BEBIG GmbH. Ophthalmic Brachytherapy 2014. 1–8. https://www.bebig.com/fileadmin/bebig_neu/user_uploads/Products/Ophthalmic_Brachytherapy/Ophthalmic_Brochure__Rev._06__English.pdf.
- Kaiserman N, Kaiserman I, Hendler K, et al. Ruthenium-106 plaque brachytherapy for thick posterior uveal melanomas. Br J Ophthalmol. 2009;93(9):1167–1171.
- Brualla L, Zaragoza FJ, Sauerwein W. Monte carlo simulation of the treatment of eye tumors with 106Ru plaques: a study on maximum tumor height and eccentric placement. Ocul Oncol Pathol. 2015;1(1):1–11.
- Filì M, Trocme E, Bergman L, et al. Ruthenium-106 versus iodine-125 plaque brachytherapy of 571 choroidal melanomas with a thickness of ≥5.5 mm. Br J Ophthalmol. 2020;104(1):26–27.
- Puusaari I, Heikkonen J, Summanen P, et al. Iodine brachytherapy as an alternative to enucleation for large uveal melanomas. Ophthalmology. 2003;110(11):2223–2234.
- Damato B, Kacperek A, Chopra M, et al. Proton beam radiotherapy of choroidal melanoma: the liverpool-clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62(5):1405–1411.
- Yarovoy AA, Magaramov DA, Bulgakova ES. The comparison of ruthenium brachytherapy and simultaneous transpupillary thermotherapy of choroidal melanoma with brachytherapy alone. Brachytherapy. 2012;11(3):224–229.
- Diaz CE, Capone A, Grossniklaus HE. Clinicopathologic findings in recurrent choroidal melanoma after transpupillary thermotherapy. Ophthalmology. 1998;105(8):1419–1424.
- Pötter R, Van Limbergen E. Uveal Melanoma. GEC ESTRO Handb. Brachytherapy. 2nd ed., 2002. p. 591–610.
- Pötter R, Van Limbergen E, Wambersie A. Reporting in brachytherapy: dose and volume specification. GEC-ESTRO Handb. Brachytherapy. 2nd ed., 2002. p. 153–215.
- Nath R, Bice WS, Butler WM, et al. AAPM recommendations on dose prescription and reporting methods for permanent interstitial brachytherapy for prostate cancer: Report of Task Group 137. Med Phys. 2009;36(11):5310–5322.
- Pötter R, Tanderup K, Kirisits C, et al. EMBRACE Collaborative Group. The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60.
- International Commission on Radiation Units and Measurements. ICRU Report 83. Prescribing, recording, and reporting photon-beam Intensity-Modulated Radiation Therapy (IMRT). J Icru. 2010;10:27–40.
- Heilemann G, Fetty L, Dulovits M, et al. Treatment plan optimization and robustness of 106Ru eye plaque brachytherapy using a novel software tool. Radiother Oncol. 2017;123(1):119–124.
- Tagliaferri L, Pagliara MM, Masciocchi C, et al. Nomogram for predicting radiation maculopathy in patients treated with Ruthenium-106 plaque brachytherapy for uveal melanoma. J Contemp Brachyther. 2017;9(6):540–547.
- Pagliara MM, Tagliaferri L, Azario L, et al. Ruthenium brachytherapy for uveal melanomas: factors affecting the development of radiation complications. Brachytherapy. 2018;17(2):432–438.
- Espensen CA, Appelt AL, Fog LS, et al. Predicting visual acuity deterioration and radiation-induced toxicities after brachytherapy for choroidal melanomas. Cancers (Basel). 2019;11(8):1124.
