?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To study the potential consequences of differences in the evaluation of variable versus uniform relative biological effectiveness calculations in proton radiotherapy for prostate cancer.
Methods and material
Experimental data with proton beams suggest that relative biological effectiveness increases with linear energy transfer. This relation also depends on the ratio, characteristic of a tissue and a considered endpoint. Three phenomenological models (Carabe et al., Wedenberg et al. and McNamara et al.) are compared to a mechanistic model based on microdosimetry (microdosimetric kinetic model) and to the current assumption of uniform relative biological effectiveness equal to 1.1 in a prostate case.
Results and conclusions
Phenomenological models clearly predict higher relative biological effectiveness values compared to microdosimetric kinetic model, that seems to approach to the constant value of 1.1 adopted in the clinics, at least for low linear energy transfer values achieved in typical prostate proton plans. All models predict a higher increase of the relative biological effectiveness-weighted dose for the prostate tumor than for the rest of structures involved due to its lower ratio, even when linear energy transfer is, in general, lower in the tumor than on the surroundings tissues. Prostate cancer is, therefore, a good candidate to take advantage of variable relative biological effectiveness, especially if linear energy transfer is enhanced within the tumor. However, the discrepancies among models hinder the clinical implementation of variable relative biological effectiveness.
Introduction
Prostate cancer is currently the second most frequent cancer disease, with more than 1,200,000 cases estimated and more than 350,000 deaths around the world only in 2018 [Citation1]. Among other treatments such as surgery, radiation therapy is considered as one of the most effective, especially for high-graded cancers [Citation2]. The most used and conventional modality is external radiotherapy with photon. Other options are also employed, such as protons or carbon ions [Citation3] in external radiotherapy or brachytherapy. In all modalities of radiation therapy, the main predictor of the subsequent effect is the amount of dose imparted to the prostate. Thus, the general objective consists of delivering enough dose to control the tumor while sparing the surrounding tissue. To achieve this goal, a number of features can be used in external radiation therapy, namely: (i) conformation of the delivered dose to the tumor by advanced techniques [Citation4]; (ii) increase of the precision of the delivery by image-guided radiotherapy [Citation5]; or (iii) different fractionation schemes of the dose by taking advantage of the variable radiobiological response of tumors and healthy tissue.
The biological response of live matter to a certain radiation dose is generally assumed to follow a Linear-Quadratic (LQ) relation as
The linear parameter,
is related to the direct damage produced by individual components of radiation beams, while
takes into account cumulative effects from interaction of the damage induced by independent radiation tracks [Citation6]. Parameters
and
or more interestingly, the ratio
are dependent on the tissue or cells [Citation7], the endpoint considered and the modality of radiation employed [Citation8], although the response is also affected by other environmental factors such as the level of oxygenation [Citation9] or immunologic mechanisms [Citation10]. In general, a high value of
indicates dominance of the track-direct action, which generally means a diminished impact of the repair processes and the cellular microenvironment. It is said then that high-
-tissues are insensitive to dose fractionation. In contrast, responses characterized by low
values markedly depend on how the dose is split. Particularly, prostate cancers seem to have low
values with respect to their adjacent normal tissue [Citation11,Citation12], which makes them an ideal candidate to increase doses per fraction [Citation13].
Modalities such as proton or carbon ion radiotherapy produce a different radiobiological response than conventional photon radiotherapy [Citation14], due to their higher linear energy transfer (LET). The way these particles deposit energy in matter is rather different from photons: they gradually lose energy as they travel through matter up to a depth at which deposit the majority of their energy and stop, producing a localized increase of dose, known as Bragg peak [Citation15]. LET is a quantity to characterize the local concentration of energy imparted along the particle track, and therefore, the LET in a particle beam increases as the particle stops. In proton therapy is usual to use dose-averaged LET (LETd), i.e. the average of the LET of each proton weighted by the dose imparted by each one of them [Citation16]. The increase of LETd at the distal side of the beam translates into a larger proportion of induced DNA double strand breaks which in turn translates into an increase of biological effectiveness [Citation17]. The ratio between the dose needed with conventional radiation (i.e. photons) and with proton or carbon ion radiation to produce a given effect is called relative biological effectiveness (RBE) [Citation18].
Based on all of the above, RBE is also expected to increase along the beam path. When multiple beams are involved in a particle treatment plan, the RBE will therefore vary across the patient anatomy [Citation19,Citation20]. However, due to the difficulties to clinically characterize RBE, and the considerable variability among in vitro and in vivo experiments, a constant value of 1.1 has been adopted for clinical use of proton therapy regardless the above mentioned increase toward the distal side of the Bragg peak [Citation21]. In this work, we assess the performance of phenomenological versus mechanistic models to predict RBE applied to the case of prostate cancer in order to evaluate the current clinical adoption and the potentiality of incorporating variable RBE to the clinics.
Methods and materials
RBE models: phenomenological versus mechanistic
Multiple predictive models for RBE have been published in the literature [Citation19,Citation22–25]. Two main categories can be distinguished: (a) phenomenological models, in which analytical expressions are obtained from fits to experimental data disregarding any information relevant to particle track structure; and (b) mechanistic models, based on the description of how energy is deposited along the particle track. As the correlation between RBE and LETd is mostly linear for the LETd values observed in proton clinical plans [Citation26], phenomenological models have been assumed to have sufficient power to predict the RBE of proton beams. It has also been argued that LETd is insufficient to describe the biological effectiveness of heavier ions, and in this case a more detailed description of the energy deposition map along the particle track is required, denoting the need of a more mechanistic approach. In general, models try to establish the correlation between RBE and its dependent variables: dose (), LETd of the radiation and
or
corresponding to the tissue and endpoint considered. It has been shown [Citation27] that a general expression for RBE is given by
(1)
(1)
where
corresponds to the limit in which
and
is the RBE corresponding to the limit in which
Usually, these values can be characterized, respectively, by
and
where the subscripts
and
indicate the parameters corresponding to protons and conventional (photon) radiations, respectively.
In this work, we select three phenomenological models well known and broadly used in the literature: the models from Wedenberg et al. [Citation22], McNamara et al. [Citation23] and Carabe et al. [Citation19]. Also, a mechanistic model is considered: the microdosimetric kinetic model [Citation28–30], that makes use of a microscopic description of the energy deposition and employs the microdosimetric analog concept of lineal energy () instead of LET [Citation31].
Description of selected phenomenological models
Each one of the phenomenological models here considered assumes different relations between the LET of the particle and the parameters and
(equivalently,
and
). Carabe et al. [Citation19] propose that both
and
increase linearly with LETd of radiation with respect to
and
respectively, and depend inverse-linearly on the ratio
of a cell line or tissue. A fit of the proposed functions to the experimental data of in vitro clonogenic survival yields the values
(2)
(2)
The assumption of in this model is based on the fact that dose fractionation effects are still present when using particles with lower LETd (such as the case of protons) [Citation32]. Wedenberg et al. [Citation22] propose the same dependencies for
but considers
i.e. the cumulative effect of tracks does not depend on LETd. From the previous assumption and a fit to experimental data and, they obtain, respectively
(3)
(3)
Finally, McNamara et al. [Citation23] consider a similar relation for but for
assume a linear decrease with both LET and
They obtain from fitting to experimental data:
(4)
(4)
Description of microdosimetric kinetic model
The Microdosimetric Kinetic Model (MKM) is based on the premise that the effects of radiation depend on the statistical distribution of specific energy (i.e. energy imparted per unit mass by independent tracks) to a sub-nuclear volume, called domain [Citation28,Citation29]. The probability of producing lethal and sub-lethal lesions are assumed to be proportional to
and sub-lethal lesions can further pair-wise interact to produce new lethal lesions. These principles are developed elsewhere [Citation28,Citation33], assuming as Wedenberg the condition
and yielding for
(5)
(5)
where
is the dose-mean specific energy, which is related to the dose-mean lineal energy
by the relation
being
the mean chord length of the tracks within the domain [Citation34];
is the density of water and
is the radius of the domain. We calculate dose, LETd and
[Citation35–37] for a clinical prostate cancer case and, using these models, RBE values are predicted to assess potential repercussions of variable effectiveness.
Radiobiological parameters for prostate cancer
In order to predict RBE for a specific tissue, besides physical calculations such as dose, LETd and it is necessary to determine the ratio
that characterizes the radiobiological response of a tissue or cell line to reference radiation (X-rays). Van Leewen et al. [Citation7] have recently published a review of parameters for cancer cells. We have taken the data from different studies for prostate cancer and have obtained an averaged weighted by the uncertainties reported by each study, obtaining a value of
1.877 Gy. As the
ratio depends on the considered endpoint, the values selected for normal tissue vary accordingly. In this study, we assume
3 Gy for all healthy tissue response, including both late rectal and recto-sigmoid complications [Citation38], and urinary toxicity (loss of frequency and capacity) [Citation39,Citation40]. Note that all models here presented are based on the evidence that RBE increases more for low
ratios. This means that, as prostate cancers have a lower
ratio than the surrounding normal tissue, their biological effectiveness is expected to be enhanced when using high-LET radiation.
Besides ratios, we need the values for the radius
of the domain characteristic of each tissue to calculate RBE according to the MKM following EquationEquation (5)
(5)
(5) . This parameter can be understood as the maximum distance through which two sub-lethal lesions can interact to form an eventual lethal lesion. We have estimated
as 488 nm by following a method detailed elsewhere [Citation41] applied to the data published by Butterworth et al. [Citation42]. We have assumed the same value for
throughout the rest of tissues involved.
We have used a clinical plan consisting of two lateral opposed scanned proton beams with a uniform dose over the target planned for each one of them. The target is the whole prostate plus the seminal vesicles, treated prophylactically, with a total prescribed dose of 70.2 Gy (physical dose) delivered in 39 fractions of 1.8 Gy each. In order to illustrate the effect of different doses per fraction, we also have calculated RBE with MKM for the same physical dose but delivered in a 3.51 Gy per fraction basis.
Results
shows an axial plane of the prostate with the calculated distribution of physical dose scaled by the clinically used value of 1.1, the distribution of LET and biological-effective dose for each one of the proposed models of variable RBE. shows metrics of the dose, LETd and distributions across the CTV and the normal-tissue organs considered in this work. Maximum values for LETd and
as high as about 10.2 keV/μm and 11.6 keV/μm, respectively, are obtained in the rectum and the bladder. These are due to the increase of these quantities at the lateral penumbra. However, mean LETd and
values are considerably lower for both structures.
Figure 1. Calculated distributions on an axial plane at the middle of the prostate of: (a) dose weighted by uniform RBE (1.1); (b) dose-averaged LET (L_D); and dose weighted by RBE according to (c) MKM; (d) Carabe et al. model; (e) Wedenberg et al. model; and (f) McNamara et al. model. The color scale for the RBE-weighted doses is the same to stress differences between variable and uniform RBE assumptions as well as among different variable-RBE models. Color scale and values at bottom left applies to RBE-weighted dose distributions and color scale and values at bottom right applies to dose-averaged LET distributions.
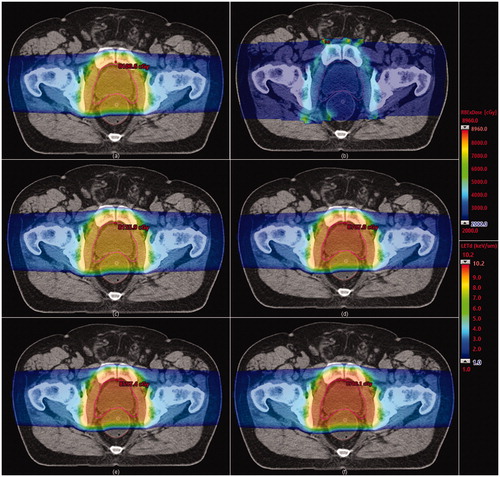
Table 1. Summary of metrics for calculated dose, dose-averaged LET and dose-mean linear energy in the relevant structures considered in this work (CTV, rectum, bladder, right femoral head and left femoral head).
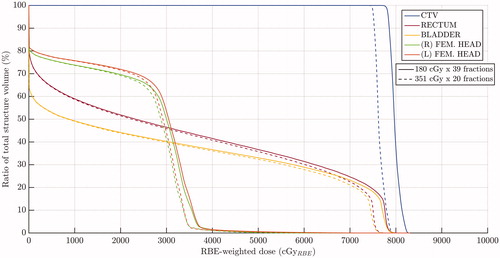
shows the dose-volume histograms of these structures for each one of these models compared to the uniform-RBE case. Note that the lower the value, the higher the increase in the increase in the RBE-weighted dose regardless the considered model. shows the results for RBE calculations taking as the references the mean doses to each structure for each considered model.
Figure 2. Dose-volume histograms calculated in terms of RBE-weighted dose for the structures considered: CTV (prostate plus seminal vesicles) ( 1.877 Gy), rectum (
3 Gy), bladder (
3 Gy) and the femoral heads (
3 Gy). Uniform RBE of 1.1 is compared to different RBE-variable models: MKM, Carabe et al., Wedenberg et al. and McNamara et al.
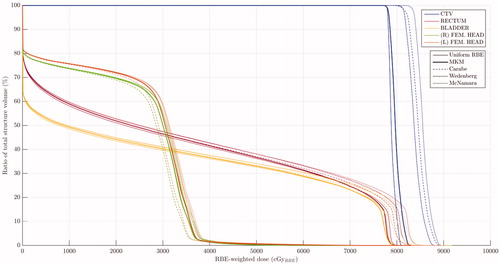
Table 2. RBE calculations according to DVHs in by using mean RBE-weighted dose to each structure as endpoint.
shows the same RBE-weighted dose calculated with MKM compared to that obtained when using a different dose per fraction (3.51 Gy/fx along 20 fractions). Mean RBE-weighted dose is reduced from 79.8 GyRBE to 76.4 GyRBE in the CTV, while it goes from 33.1 GyRBE to 32.0 GyRBE in the rectum; from 28.8 GyRBE to 27.8 GyRBE in the bladder; from 23.1 GyRBE to 22.1 GyRBE in the right femoral head; and from 24.0 GyRBE to 22.9 GyRBE in the left femoral head.
Discussion
As and show, all phenomenological variable-RBE models predict a clear increase of the biological effectiveness with respect to the current clinical assumption of uniform RBE set to 1.1. However, the MKM points toward a slight increase from the uniform value in both CTV and OARs such as rectum and bladder. Even though all phenomenological models show an increase of the RBE to the tumor, there are still appreciable differences among them, ranging from 6% to 8% in mean RBE-weighted dose. On the other hand, as shown in , LET tends to increase at the distal edge of the beam and at the lateral penumbra. The LET distribution shows maximum value around 10 keV/μm in the rectum or in the bladder, clearly higher than the order of the values in the CTV. However, the relatively low dose imparted to those areas of high LET contain their significance in terms of RBE-weighted dose volume histogram, as shown in . Interestingly, for structures with low ratios, the relative enhancement on RBE-weighted dose predicted by any model is larger than for structures with high
ratios, even when LET is not considerably high.
Consequently, for the prostate case, the key seems to reside in the ratios. Note that, with respect to the current adoption of constant RBE, phenomenological models suggest an increase of both tumor control probability (TCP) and normal tissue complication probability (NTCP), greater for TCP because of an enhancement of the dose to the tumor, while the MKM predicts a more modest increase of TCP and small differences in NTCP. Hence, whereas phenomenological models suggest an actual widening of the therapeutic window, the mechanistic MKM, yet showing the same effect, tends to soften its impact. As it can be inferred from EquationEquation (1)
(1)
(1) , RBE tends to decrease as dose per fraction increases. In other words, as shown in , hypo-fractionated schemes, which also are indicated for cases of
ratios lower for tumors than for normal tissue, play against any therapeutic advantage indicated by the variable-RBE effect here shown. Indeed, when going from 1.8 Gy/fx to 3.51 Gy/fx keeping the same total physical dose, RBE-weighted mean dose decreases by 3.1 GyRBE in the CTV while it does in the order of 1 GyRBE in the organs at risk considered. However, hypofractionation itself tends to increase the biological effective dose (BED) regardless the type of radiation (X-rays or protons). The smaller the
ratio, the greater BED, so that the prostate characteristics enable a potential advantage for hypofractionated schemes in X-ray therapy. Hence, BED and RBE are competitive effects when changing the fractionation scheme in proton therapy. In other words, the potential radiobiological advantage for protons over X-ray fades away toward hypofractionation. One potential clinical application of proton therapy refers to cases in which hypo-fractionation is not possible with conventional therapy.
A higher RBE has been reported when LET values are increased within the target [Citation43]. Heavy ions increase the values of LET, although not only in the tumor. Arc proton therapy might perform this task [Citation44,Citation45] by using multiple Bragg peaks coming from different angles positioned at the center of the tumor. This technique not only increases LET in the tumor but also makes it lower in the rest of structures. Therefore, the observed differences in between the constant-RBE hypothesis and variable-RBE models would be augmented.
Nevertheless, a major flaw of the variable-RBE paradigm is also visible in . The considered models here present large differences among themselves, making uncertain the clinical decision. In this work we have compared three phenomenological models with the mechanistic MKM. All of them seem to overestimate the RBE compared to the MKM for the CTV structure and high dose regions, while Carabe et al. seem to underestimate the RBE for low dose regions. In any case, further investigation is needed to gain knowledge on the biological response as well as accuracy on the RBE prediction to eventually bring the potential advantages to the clinics.
Conclusions
The current clinical consensus on radiotherapy treatments with protons considers a single, constant value, 1.1, for the relative biological effectiveness of this modality. However, growing in vitro and in vivo experimental data suggest an increase of the RBE with high LET. This increase also depends on the ratio of the considered tissue and endpoint, and it is particularly large when for low
ratios. This is potentially exploitable for prostate cancer, in which generally tumors have lower
ratios than the adjacent tissue. However, phenomenological RBE models seem to overestimate these effects compared to the mechanistic MKM. This large variability among RBE models challenges the clinical implementation of variable RBE.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Culp MBB, Soerjomataram I, Efstathiou JA, et al. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77(1):38–52.
- Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532–2542.
- Ishikawa H, Tsuji T, Kamada , the Working Group for Genitourinary Tumors, et al. Carbon-ion radiation therapy for prostate cancer. Int J Urol. 2012;19(4):296–305.
- Guckenberger M, Flentje M. Intensity-modulated radiotherapy (IMRT) of localized prostate cancer: a review and future perspectives. Strahlenther Onkol. 2007;183(2):57–62.
- Zelefsky MJ, Kollmeier M, Cox B, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84(1):125–129.
- McMahon SJ. The linear quadratic model: Usage, interpretation and challenges. Phys Med Biol. 2018;64(1):01TR01–01TR24.
- van Leeuwen CM, Oei AL, Crezee J, et al. The alpha and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat. Oncol. 2018;13(1):1–11.
- Joiner M, van der Kogel A. Basic clinical radiobiology. 4th ed. London, UK: Hodder Education; 2009.
- Yaromina A, Thames H, Zhou X, et al. Radiobiological hypoxia, histological parameters of tumour microenvironment and local tumour control after fractionated irradiation. Radiother. Oncol. 2010; 96(1):116–122.
- Siva S, MacManus MP, Martin RF, et al. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356(1):82–90.
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43(5):1095–1101.
- Fowler J, Chappell R, Ritter M. Is α/β for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50(4):1021–1031.
- Chen LN, Suy S, Uhm S, et al. Stereotactic Body Radiation Therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. 2013;8(1):1–10.
- Fossati P, Matsufuji N, Kamada T, et al. Radiobiological issues in prospective carbon ion therapy trials. Med Phys. 2018;45(11):e1096–e1110.
- Paganetti H. Proton therapy physics. Boca Raton, FL: CRC Press - Taylor & Francis Group; 2012.
- Grassberger C, Trofimov A, Lomax A, et al. Variations in linear energy transfer within clinical proton therapy fields and the potential for biological treatment planning. Int J Radiat Oncol Biol Phys. 2011;80(5):1559–1566.
- Stewart RD, Yu VK, Georgakilas AG, et al. Effects of radiation quality and oxygen on clustered DNA lesions and cell death. Radiat Res. 2011;176(5):587–602.
- Paganetti H, Blakely E, Carabe-Fernandez A, et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys. 2019;46(3):e53–e78.
- Carabe A, España S, Grassberger C, et al. Clinical consequences of relative biological effectiveness variations in proton radiotherapy of the prostate, brain and liver. Phys Med Biol. 2013;58(7):2103–2117.
- Pedersen J, Petersen JBB, Stokkevåg CH, et al. Biological dose and complication probabilities for the rectum and bladder based on linear energy transfer distributions in spot scanning proton therapy of prostate cancer. Acta Oncol. (Madr). 2017; 56(11):1413–1419.
- Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59(22):R419–R472.
- Wedenberg M, Lind BK, Hårdemark B. A model for the relative biological effectiveness of protons: the tissue specific parameter α/β of photons is a predictor for the sensitivity to LET changes. Acta Oncol. (Madr). 2013;52(3):580–588.
- McNamara AL, Schuemann J, Paganetti H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys Med Biol. 2015;60(21):8399–8416.
- Frese MC, Yu VK, Stewart RD, et al. A mechanism-based approach to predict the relative biological effectiveness of protons and carbon ions in radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83(1):442–450.
- Carlson DJ, Stewart RD, Semenenko VA, et al. Combined use of Monte Carlo DNA damage simulations and deterministic repair models to examine putative mechanisms of cell killing. Radiat. Res. 2008;169(4):447–459.
- Durante M, Paganetti H, Kry SF, et al. Report of a National Cancer Institute special panel: characterization of the physical parameters of particle beams for biological research. Med Phys. 2019;46(2):e37–e52.
- Carabe A, Moteabbed M, Depauw N, et al. Range uncertainty in proton therapy due to variable biological effectiveness. Phys Med Biol. 2012;57(5):1159–1172.
- Hawkins RB. A microdosimetric-kinetic model of cell death from exposure to ionizing radiation of any LET, with experimental and clinical applications. Int J Radiat Biol. 1996;69(6):739–755.
- Hawkins RB. A microdosimetric-kinetic model for the effect of non-poisson distribution of lethal lesions on the variation of RBE with LET. Radiat Res. 2003;160(1):61–69.
- Kase Y, Kanai T, Matsufuji N, et al. Biophysical calculation of cell survival probabilities using amorphous track structure models for heavy-ion irradiation. Phys Med Biol. 2008;53(1):37–59.
- Bertolet A, Cortés‐Giraldo MA, Carabe‐Fernandez A. On the concepts of dose-mean lineal energy, unrestricted and restricted dose-averaged LET in proton therapy. Phys Med Biol. 2020;65(7):075011.
- Carabe-Fernandez A, Dale RG, Hopewell JW, et al. Fractionation effects in particle radiotherapy: Implications for hypo-fractionation regimes. Phys Med Biol. 2010;55(19):5685–5700.
- Kase Y, Kanai T, Matsumoto Y, et al. Microdosimetric measurements and estimation of human cell survival for heavy-ion beams. Radiat Res. 2006;166(4):629–638.
- Kellerer AM. Fundamentals of microdosimetry. In: Kase KR, Bjarngard BE, Attix FH, editors. The dosimetry of ionization radiation. Vol. I. Orlando, FL: Academic Press, Inc.; 1985. pp. 77–162.
- Bertolet A, Cortés-Giraldo MA, Souris K, et al. Calculation of clinical dose distributions in proton therapy from microdosimetry. Med Phys. 2019;46(12):5816–5823.
- Bertolet A, Baratto‐Roldán A, Cortés‐Giraldo MA, et al. Segment-averaged LET concept and analytical calculation from microdosimetric quantities in proton radiation therapy. Med Phys. 2019;46(9):4204–4214.
- Bertolet A, Cortés‐Giraldo MA, Souris K, et al. A kernel‐based algorithm for the spectral fluence of clinical proton beams to calculate dose‐averaged LET and other dosimetric quantities of interest. Med Phys. 2020. DOI:10.1002/mp.14108
- Marzi S, Saracino B, Petrongari MG, et al. Modeling of α/β for late rectal toxicity from a randomized phase II study: conventional versus hypofractionated scheme for localized prostate cancer. J Exp Clin Cancer Res. 2009;28(1):1–8.
- Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low α/β ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52(1):6–13.
- Franco RD, Borzillo V, Ravo V, et al. Rectal/urinary toxicity after hypofractionated vs conventional radiotherapy in low/intermediate risk localized prostate cancer: systematic review and meta analysis. Oncotarget. 2017;8(10):17383–17395.
- Bertolet A, Cortés‐Giraldo MA, Carabe‐Fernandez A. Analysis of a new method to determine domain sizes to calculate RBE in proton therapy with the microdosimetric kinetic model using analytical calculations of dose-mean linear energy in a TPS. Phys Med Biol. 2020.
- Butterworth KT, McGarry CK, Clasie B, et al. Relative biological effectiveness (RBE) and out-of-field cell survival responses to passive scattering and pencil beam scanning proton beam deliveries. Phys Med Biol. 2012;57(20):6671–6680.
- Fager M, Toma-Dasu I, Kirk M, et al. Linear energy transfer painting with proton therapy: a means of reducing radiation doses with equivalent clinical effectiveness. Int J Radiat Oncol. 2015;91(5):1057–1064.
- Carabe-Fernandez A, Bertolet A, Karagounis IV, et al. Is there a role for arcing techniques in proton therapy? BJR. 2020;93(1107):20190469.
- Bertolet A, Cortés-Giraldo MA, Carabe-Fernandez A. Proton Monoenergetic Arc Therapy (PMAT) to enhance LETd within the target. Phys Med Biol. 2020.