ABSTRACT:
Background: Comorbidity is an important prognostic marker and a treatment indicator for lung cancer patients. Register-based studies often describe the burden of comorbidity by the Charlson comorbidity index (CCI) based on hospital discharge data. We assessed the association between somatic and psychiatric comorbidity and death within one year in early lung cancer and, furthermore, the burden of comorbidity according to treatment type.
Material and methods: We conducted a population-based matched case-control study of stage I lung cancer identifying all treated patients who died (all-cause) within one year after diagnosis (early death group, cases). On the basis of data from the Danish Lung Cancer Registry these patients were then matched with two controls who survived more than one year (survivors). Through a review of the medical records, we validated inclusion criteria and collected data on somatic and psychiatric comorbidity. We assessed the association between comorbidity and early death with multivariate conditional logistic regression.
Results: We included 221 cases and 410 controls. The mean CCI score in the early death group was 2.3 vs. 1.3 in the survivor group (p < .001). Still, 22% vs. 30% had a CCI score of zero (p = .04) with an average number of comorbidities among these patients of 1.63 vs. 1.06 respectively (p = .006). Among women, 23% in the early death group had depression vs. 13% in the survivor group, corresponding to an unadjusted odds ratio (OR) of 2.0 (CI 95% 1.0–3.7). However, in an adjusted analysis (incl. somatic comorbidities) the OR was 1.7 (CI 95% 0.8–3.5). Patients undergoing oncological therapy were older and tended to have more somatic comorbidities than the surgically treated patients.
Conclusion: Comorbidity remains a significant prognostic marker even for stage I lung cancer patients with a CCI score of zero. The suggested association between early death and depression among women needs to be studied further.
Introduction
Lung cancer is globally the most common cause of cancer-related death [Citation1]. Consequently, several initiatives to improve the prognosis have been undertaken. These have focussed on early identification, while also the quality and number of treatment options have gradually increased [Citation2,Citation3]. Thus, a larger proportion of medically unfit lung cancer patients can now receive curative treatment [Citation4]. Longstanding tobacco smoking is the main risk factor for lung cancer, and is also related to the development of several comorbidities [Citation5–7]. The impact of comorbidity on lung cancer prognosis is, however, difficult to establish. Aside from the direct effect on prognosis, comorbidity also indirectly affects both treatment decisions and the occurrence of treatment complications [Citation8–10]. Furthermore, lung cancer patients with comorbidities may benefit from a favourable stage distribution due to early detection [Citation11]. Among several comorbidity indexes, the Charlson comorbidity index (CCI) [Citation12–14] has often been used to describe the burden of comorbidity as a prognostic marker in population-based studies. In these studies, the burden of comorbidities is often assessed by hospital discharge diagnoses. However, large proportions of patients in these lung cancer populations have a CCI score of zero [Citation15,Citation16]. Furthermore, the CCI measures exclusively somatic comorbidity while psychiatric disorders may also affect the prognosis for cancer patients [Citation17]. One- and five year mortality rates are common landmarks when assessing cancer related outcomes. Our aim was to focus on early unanticipated death. Thus, in an explorative population-based matched case-control study, we have assessed the impact of both somatic and psychiatric comorbidities in relation to one-year mortality and treatment decisions for Danish stage I lung cancer patients who were considered candidates for curative therapy at the time of final staging.
Material and method
This is a population-based nested case-control study. Outcome was all-cause one-year mortality and treatment decision.
Data sources
The Danish Lung Cancer Registry (DLCR) has since 1 January 2000 collected data on all incident lung cancer patients in Denmark including information on several lung cancer and patient-specific clinicopathological parameters [Citation18].
The Danish Cancer Registry (DCR) has collected data on all incident malignant, premalignant, and certain benign neoplasms since 1943 [Citation19]. Data on type and year of diagnosis of another primary cancer diagnosed prior to or during time of diagnosis of the lung cancer were retrieved from the DCR.
Medical records from all included patients from the departments of pulmonology, oncology, and thoracic surgery, were used to retrieve detailed data concerning the study variables.
Study population
We identified patients in the DLCR with stage I lung cancer diagnosed between 1 January 2011 and 31 December 2014, with a treatment registration who were referred to curative treatment and died within one year after diagnosis. These were then matched by age (five-year increments) and gender with two controls diagnosed the same or previous year and who survived more than one year.
Ethical approval
The study was approved by the Danish Data Protection agency and The Danish Health Authority.
Study variables
Data on comorbidities were primarily retrieved from the summary of the medical history including daily medications, which is mandatory to document in the medical record at first visit. Comorbidities uncovered during the diagnostic work-up for lung cancer were also included. We chose this approach since treatment decisions were based alone on these data.
Comorbidity, both somatic and psychiatric was defined as a chronic systemic or vital organ disease, condition or malignancy diagnosed prior to or revealed during the diagnostic work-up for the primary lung cancer. Ongoing acute infections, exacerbations, procedure-related complications, osteoarthritis, and pain syndromes that were not attributed to a systemic condition were not included. Moreover, pain medication, preventive medications (e.g., statins), short duration antibiotics, and corticosteroids were not included in the assessment of the daily medication.
Psychiatric comorbidity was categorised as dementia, depression (including bipolar conditions), or schizophrenia (including other psychotic conditions).
Malignant comorbidities were grouped according to ICD-10 codes as follows: upper gastrointestinal (upper GI), DC00-DC14; lower GI, DC15.-DC26; respiratory and other intra-thoracic malignancies DC30-DC39; breast DC50; female genitalia DC51-DC58; prostate cancer DC61; haematologic DC81-96; other malignancies, any other DCXX (with the exception of non-melanoma skin cancer). Malignancies diagnosed prior to 1985 and precancerous conditions (DDXX) were not included. Drawing on medical records, we established the remission status of the prior primary cancer at time of the index lung cancer. If the diagnostic work-up for the other cancer was ongoing or the patient had not yet received a planned treatment (curative or palliative) the other cancer was registered as not in remission. Cancers that were under ‘watchful waiting’ or active surveillance were registered as not in remission. From the medical records we also established the CCI-score for each patient.
Concerning lung cancer treatment, we included only patients who were referred to curative treatment at time of diagnosis. If treatment intent changed after the point of referral, patients were still included. Based on the medical records, we registered the actual treatment given as either surgical or oncological, which was further categorised into either stereotactic body radiotherapy (SBRT) or other, (conventional chemo/radiotherapy, palliative radiotherapy and systemic treatment).
High-risk alcohol intake was defined as more than 14 or 21 units per week for women and men, respectively, in accordance with the recommendations from the Danish Health Authority at the start of the study period [Citation20]. Smoking was categorised as ever-smoker, never-smoker, or unknown at diagnosis.
Statistical analyses
Proportional and Pearson chi squared distributions were used to compare categorical variables between the two groups. For continuous variables, the Mann–Whitney U-test was used. Provided that the Q-Q plotted data followed an approximated normal distribution, the mean age in the study population(s) and the underlying DLCR- cohort were compared by the student’s t-test.
Associations between the study variables and outcome (death within a year after diagnosis) were assessed by using a conditional logistic regression model. Each disease was assessed as a binary variable in the model. All analyses were conducted for the total sample and by gender. In order to assess the effect of the combined comorbidities in relation to outcome and the effect of somatic comorbidities on clinical depression, continuous variables were created by combining the total number of comorbidities, the total number of somatic comorbidities and the number of different types of daily prescription medications, not the number of administrations.
For each treatment group, we assessed differences in age and gender among the early death patients. Within each treatment group, we assessed differences in the number of non-malignant and somatic comorbidities, the number of daily medications, the proportion of patients with depression (combined and by gender), and the burden of malignant comorbidity, between the early death patients and survivors
Calculations were performed with SAS software (SAS system, SAS Institute, Cary, NC) and Stata software (StataCorp, 4905 Lakeway Drive College Station, Texas 77845 USA).
Results
According to the DLCR, 2985 patients (DLCR cohort) were diagnosed with stage I lung cancer in Denmark between 2011 and 2014, with a one-year mortality of 13% (n = 382), among whom 269 patients had a treatment registration. We were able to match 253 of the treated patients (early death) with two controls. After review of the medical records, we excluded patients considered candidates for treatment with palliative intent, patients who did not have lung cancer, or had an incorrect stage classification. We finally included 221 patients in the early death group and 410 in the survivor group, baseline characteristics are provided in .
Table 1. Baseline characteristics of the early death group vs. the survivor group, sampled from the Danish stage I lung cancer cohort in the Danish Lung Cancer Register (2011–2014).
Treatment and age
In the early death group, 127 (57%) were surgically treated and 94 (43%) were treated oncologically (78 with SBRT and 16 with conventional therapy, of whom eight ultimately received only palliative therapy, in spite of initial curative intent). In these two treatment groups, we compared the age mean in the early death group with the underlying DLCR cohort. The mean age among the 2021 surgically treated patients in the DLCR cohort was 67.5 vs. 71.7 among the surgically treated early death patients (p < .001). Conversely, there was no statistically significant difference in age between the oncologically treated patients in the DLCR cohort (n = 663), where the mean age was 73.5 vs. 75.0 in the early death group (p = .12).
Comorbidity and CCI
The CCI score was significantly higher in the early death group (). Still, 48 (22%) of patients who died early and 121 (30%, p = .04) in the survivor group had a CCI score of zero (data not shown). In this subgroup of patients with a CCI of zero, we summarised the number of comorbidities recorded in the medical records with a mean number of 1.63 vs. 1.06, respectively (p = .006). For somatic comorbidities, this number was 1.56 vs. 0.96, respectively (p = .002).
Table 2. Prevalence of comorbidity at time of diagnosis and the odds ratio for death within 1 year after diagnosis of stage I lung cancer, Denmark 2011–2014.
In the adjusted analysis of specific comorbid conditions, atrial fibrillation/flutter, valvulopathy, peripheral vascular disease, and peptic ulcer were significantly associated with one-year mortality (). In the gender-stratified analyses, diabetes was statistically significantly associated with one-year mortality among women but not in men.
As appears in , women in the early death group had a significantly higher number of comorbidities and took more medications than the survivors did while this was not observed among men.
Table 3. Cumulated number of comorbidities, medications and the associations with the early death among stage I lung cancer patients, Denmark, 2011–14.
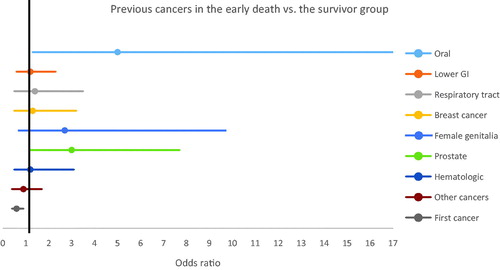
Malignant comorbidity
In terms of site-specific cancers, the odds ratio for previous oral or prostate cancer was significantly higher in the early death group (). Twenty-three patients (10%) in the early death group vs. 14 (3%) among the survivors were living with a cancer that was not in remission when they were diagnosed with lung cancer. This corresponded to an unadjusted OR of 3.0 (CI 95% 1.5–5.9), and an adjusted OR of 3.3 when adjusted for non-malignant comorbidities (CI 95% 1.7–6.6) (data not shown). Furthermore, the OR for lung cancer as first cancer was significantly lower in the early death group ().
Comorbidity by treatment modality
Among patients treated with surgery, there was no significant difference in the number of non-malignant comorbidities, somatic comorbidities, and daily medication between the early death group and the survivor group (). However, more patients in the early death group had a cancer prior to the lung cancer diagnosis (p = .03) and more patients had progression of their second cancer (p = .001).
Table 4. Distribution of age, comorbidities and depression according to type of treatment, all patients were considered candidates for curative therapy after final staging.
Regarding the oncologically managed patients, the early death group had a higher number of comorbidities and were more medicated than the survivors were. Furthermore, there was no difference in the prevalence of previous cancer between the early death group and the survivors among the oncologically managed.
Psychiatric comorbidity
As seen in , depression was significantly associated with early death among women with an adjusted OR of 2.1 (CI 95% 1.1–4.1). Due to potential confounding by somatic comorbidity, we adjusted for somatic comorbidity and other primary cancer in the gender-stratified conditional regression model, resulting in an OR of 1.7 (CI 95% 0.8–3.5).
In terms of depression and treatment type (), there was a trend in both treatment groups of depression occurring more frequently among women in the early death group ().
None of the other psychiatric comorbidities, which occurred with a much lower prevalence than depression, were associated with early death ().
Discussion
The cause of death among early death patients has been reported earlier [Citation21]. Furthermore, patients with no treatment registration in the DLCR have been assessed in another study [Citation22].
It is beyond the scope of this article to discuss each association individually, although we have reported a number of important findings in terms of individual comorbidities (atrial fibrillation and valvulopathy), which were individually associated with early death, even though these diseases are not included in the CCI. Atrial fibrillation in relation to lung cancer has primarily been studied as a complication following lung cancer surgery [Citation23,Citation24]. Moreover, the association with prognosis may be facilitated by coexisting COPD as suggested in a retrospective single-center study by Sekine et al. from 2001 of 244 surgically treated patients [Citation25].
Liver disease, primarily alcoholic cirrhosis of the liver, was associated with early death, although the risk estimate was weakened in the multivariate analysis, suggesting that this association was partly confounded by high-risk alcohol intake and smoking [Citation26]. There have been few studies regarding cirrhosis and lung cancer [Citation27] but our findings suggest that although relatively infrequent, alcohol-related comorbidity was significant in our population and that differences in alcohol consumption between countries may contribute to differences in survival rates for (early stage) lung cancer [Citation28].
In a study based on the DLCR of comorbidity in lung cancer, comprising 20,461 patients diagnosed in 2005–2010, a high Charlson Comorbidity Index score (CCI > 3) based on hospital contacts registered in the National Patient Registry was related to mortality. However, in that study 10,289 (50%) patients, of whom 1653 were resected, had a CCI score of zero. These patients had a one-year mortality of 11%. In our study, even among these, patients with a CCI of zero, the early death patients still had a significantly higher number of comorbidities than the survivors. Moreover, of 631 patients reviewed, none had AIDS, or hemiplegia; these diseases therefore appeared to have a low prevalence in our population, which is supported by a Swedish register-based case/non-case control lung cancer study by Nilsson et al. [Citation29].
Among women, the prevalence of depression was 23 vs. 13%, in the early death and survivor group, respectively, with a 2.1-fold significantly increased adjusted OR. This association seemed to be partly confounded or potentially mediated by the prevalence of somatic diseases, as the risk estimate was slightly lower at 1.7-fold increase after adjustment and failed to reach statistical significance. There was no indication in our study population that patients with depression chose or were offered oncological treatment over surgery. Furthermore, we registered only one suicide in a male patient. Former studies have also found an association between depression and unfavourable outcome [Citation30], with a suggested mechanism of continued smoking and lacking ability to achieve sustained tobacco abstinence [Citation31]. However, in addition to this, our findings underscore the importance of a meticulous assessment of somatic comorbidities when assessing the prognostic significance of depression.
Tobacco smoking increases the risk of developing other cancers. Thus patients with multiple cancers are more prevalent among lung cancer patients than other primary (non smoking-related) cancers [Citation32]. As also reflected in several predictive analyses and comorbidity indexes (e.g., the CCI) [Citation12,Citation13], co-occurrence of other primary cancer disease, was also associated with a poor outcome in our population, since there were significantly more patients in the early death group with another cancer, particularly patients who had an non-remission secondary cancer. The association between oral cancer and early death could be due to increased alcohol intake among these patients [Citation33]. The association between prostate and early death cannot readily be explained and our findings need to be confirmed in other studies. In terms of other cancers there were a trend towards an association with early death.
Given increased and improved treatment options for several cancers, patients with multiple cancers will continue to constitute significant clinical challenges. The fact that patients are referred to curative treatment in spite of having a second cancer is reflective of treatment optimism. However, based on our findings, maybe these patients should be referred to non-surgical treatment ().
The cumulated number of non-malignant comorbidities and medications was significantly associated with early death among female patients but not in men. An American single-center study of 1155 lung cancer patients diagnosed between 1995 and 1998 by Tammemagi et al. [Citation34], reported that a high comorbidity count, male gender, and alcohol were associated with a poor prognosis. The number of comorbid disorders was not associated with early death among male patients in our patients, which may be indicative of an overall higher burden of comorbidities among Danish men with early stage lung cancer.
As expected, oncological treatment was associated with advanced age and an overall high burden of comorbidity [Citation29,Citation35,Citation36]. Moreover, the number of non-malignant comorbidities was significantly associated with early death among patients undergoing oncological treatment whereas malignant comorbidity was not. However, as mentioned earlier, among the surgically treated, malignant comorbidity was significantly associated with early death.
Strengths and limitations
In the present population-based study, we assessed the association between the burden of comorbidity assessed at time of diagnosis using data at hand for the clinicians involved in treatment of the individual patient. By including only stage I patients, we have selected patients with the a priori most favourable prognosis with regard to the lung cancer itself, so that, arguably, these patients could be those most sensitive to the negative consequences of comorbidity. Moreover, by including only stage I lung cancer patients, we avoid confounding by the favourable stage distribution formerly described [Citation11].
Our study has a number of limitations. The case-control design has enabled us to assess several comorbidities, but given the matching criteria (i.e., age and gender) we may have high internal validity but less generalizability in the assessment of treatment modality in relation to the burden of comorbidity. For instance, we have only included patients with a treatment registration in the DLCR, and it is plausible that among patients who were not treated at all, the burden of comorbidity would be even more strongly associated with early death. Thus, our findings can only be related to patients who were considered candidates for curative therapy at the time of referral to treatment.
We based our assessment of the non-malignant comorbidities, daily medications, remission status (but not type) of other primary cancer, and alcohol and smoking habits solely on the medical records and failure in recording a given entity may have resulted in misclassification. This may in particular apply for comorbidities that have not traditionally been linked to the prognosis of lung cancer. However, by including medications and their related indications in the assessment of the burden of comorbidity, we have minimised this potential source of misclassification. This method may, however, have resulted in misclassification of comorbidities based on medications for which there were more than one indication. However, as all data regarding the study variables were collected at the same point during the course of the disease, we believe that any misclassification was non-differential and did not alter the directions of the associations.
Conclusion
Somatic comorbidity, both malignant and non-malignant, was significantly associated with early death in patients with stage I lung cancer. Having another primary cancer under treatment at time of diagnosis of the lung cancer was the condition most strongly associated with early death. Comorbidities that are not included in the often used Charlson Comorbidity Index were also associated with death within the first year. Women with more than two comorbidities constitute a subgroup of patients with an adverse outcome. Furthermore, depression was associated with early death among women. This association seemed partly due to co-occurrence with somatic comorbidities and further studies into this area are warranted. Our findings indicate that clinicians should include conditions like atrial fibrillation/flutter and valve disease as well as depression in their prognostic assessment of early stage lung cancer patients.
Disclosure statement
The authors have no conflicts of interest in relation to the present study.
References
- Wong MCS, Lao XQ, Ho K-F, et al. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7(1):14300.
- Ceniceros L, Aristu J, Castañón E, et al. Stereotactic body radiotherapy (SBRT) for the treatment of inoperable stage I non-small cell lung cancer patients. Clin Transl Oncol. 2016;18(3):259–268.
- Bi N, Shedden K, Zheng X, et al. Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable stage I non-small cell lung cancer: a systemic review and pooled analysis. Int J Radiat Oncol. 2016;95(5):1378–1390.
- Ezer N, Veluswamy RR, Mhango G, et al. Outcomes after stereotactic body radiotherapy versus limited resection in older patients with early-stage lung cancer. J Thorac Oncol. 2015;10(8):1201–1206.
- U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta (GA): Centers for Disease Control and Prevention; 2004.
- Peto R, Darby S, Deo H, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329.
- Taghizadeh N, Vonk JM, Boezen HM. Lifetime smoking history and cause-specific mortality in a cohort study with 43 years of Follow-up. PLoS One. 2016;11(4):e0153310–18.
- Simonsen DF, Søgaard M, Bozi I, et al. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med. 2015;109(10):1340–1346.
- Islam KMM, Jiang X, Anggondowati T, et al. Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1079–1085.
- Rios J, Gosain R, Goulart BHL, et al. Treatment and outcomes of non-small-cell lung cancer patients with high comorbidity. CMAR. 2018;10:167–175.
- Janssen-Heijnen ML, Schipper RM, Razenberg PP, et al. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. 1998;21(2):105–113.
- Ganti AK, Siedlik E, Marr AS, et al. Predictive ability of Charlson Comorbidity Index on outcomes from lung cancer. Am J Clin Oncol. 2011;34(6):593–596.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Thygesen SK, Christiansen CF, Lash TL, et al. Predictive value of coding of diagnoses in the charlson comorbidity index in the Danish national registry of patients. Pharmacoepidemiol Drug Saf. 2009;18(S1):S189.
- Lüchtenborg M, Morris EJA, Tataru D, et al. Investigation of the international comparability of population-based routine hospital data set derived comorbidity scores for patients with lung cancer. Thorax. 2018;73(4):339–349.
- Parés-Badell O, Banqué M, Macià F, et al. Impact of comorbidity on survival by tumour location: Breast, colorectal and lung cancer (2000-2014). ).Cancer Epidemiol. 2017;49:66–74.
- Suppli NP, Johansen C, Kessing LV, et al. Survival after early-stage breast cancer of women previously treated for depression: a nationwide Danish cohort study. J Clin Oncol. 2017;35(3):334–342.
- Jakobsen E, Green A, Oesterlind K, et al. Nationwide quality improvement in lung cancer care: the role of the Danish Lung Cancer Group and Registry. J Thorac Oncol. 2013;8(10):1238–1247.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–45.
- Danish Health Authority. [cited 2020 May 14]. Available form: https://www.sst.dk/en/health-and-lifestyle/alcohol.
- Christensen N, Kejs AM, Jakobsen E, et al. Early death in Danish stage I lung cancer patients. A population-based case study. Acta Oncol (Madr). 2018;57(11):1561–1566.
- Christensen NL, Dalton S, Ravn J, et al. Treatment, no treatment and early death in Danish stage I lung cancer patients. Lung Cancer. 2019;131:1–5.
- Onaitis M, D'Amico T, Zhao Y, et al. Risk factors for atrial fibrillation after lung cancer surgery: Analysis of the society of thoracic surgeons general thoracic surgery database. Ann Thorac Surg. 2010;90(2):368–374.
- Imperatori A, Mariscalco G, Riganti G, et al. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. 2012;7(1):1–8.
- Sekine Y, Kesler KA, Behnia M, et al. COPD may increase the incidence of refractory supraventricular arrhythmias following pulmonary resection for non-small cell lung cancer. Chest. 2001;120(6):1783–1790.
- Christensen NL, Løkke A, Dalton SO, et al. Smoking, alcohol, and nutritional status in relation to one-year mortality in Danish stage I lung cancer patients. Lung Cancer. 2018;124:40–44.
- De Goede B, Klitsie PJ, Lange JF, et al. Morbidity and mortality related to non-hepatic surgery in patients with liver cirrhosis: a systematic review. Best Pract Res Clin Gastroenterol. 2012;26(1):47–59.
- Walters S, ICBP Module 1 Working Group, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax. 2013;68(6):551–564.
- Nilsson J, Berglund A, Bergström S, et al. The role of comorbidity in the management and prognosis in nonsmall cell lung cancer: a population-based study. Acta Oncol. 2017;56(7):949–956.
- Vodermaier A, Lucas S, Linden W, et al. Anxiety after diagnosis predicts lung cancer-specific and overall survival in patients with stage III non-small cell lung cancer: a population-based cohort study . J Pain Symptom Manage. 2017;53(6):1057–1065.
- Cooley ME, Wang Q, Johnson BE, et al. Factors associated with smoking abstinence among smokers and recent-quitters with lung and head and neck cancer. Lung Cancer. 2012;76(2):144–149.
- Vogt A, Schmid S, Heinimann K, et al. Multiple primary tumours : challenges and approaches, a review. 2017;2:e000172.
- Schildt E, Eriksson M, Hardell L, et al. Oral snuff, smoking habits and alcohol consumption in relation to oral cancer in a Swedish case-control study. Int J Cancer. 1998;77(3):341–346.
- Tammemagi CM, Neslund-Dudas C, Simoff M, et al. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol. 2004;57(6):597–609.
- Iachina M, Green A, Jakobsen E. The direct and indirect impact of comorbidity on the survival of patients with non-small cell lung cancer: a combination of survival, staging and resection models with missing measurements in covariates. BMJ Open. 2014;4(2):e003846.
- Mokhles S, Nuyttens JJ, Maat A, et al. Survival and treatment of non-small cell lung cancer stage I – II treated surgically or with stereotactic body radiotherapy: patient and tumor-specific factors affect the prognosis. Ann Surg Oncol. 2015;22(1):316–323.