?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Spot-scanning proton arc therapy (SPArc) has been proposed to improve dosimetric outcome and to simplify treatment workflow. To efficiently deliver a SPArc plan, it’s crucial to minimize the number of energy layer switches (ELS) a sending because of the magnetic hysteresis effect. In this study, we introduced a new SPArc energy sequence optimization algorithm (SPArc_seq) to reduce ascended ELS and to investigate its impact on the beam delivery time (BDT).
Method and materials
An iterative energy layer sorting and re-distribution mechanism following the direction of the gantry rotation was implemented in the original SPArc algorithm (SPArc_orig). Five disease sites, including prostate, lung, brain, head neck cancer (HNC) and breast cancer were selected to evaluate this new algorithm. Dose-volume histogram (DVH) and plan robustness were used to assess the plan quality for both SPArc_seq and SPArc_orig plans. The BDT evaluations were analyzed through two methods: 1. fixed gantry angle delivery (BDTfixed) and 2. An in-house dynamic arc scanning controller simulation which considered of gantry rotation speed, acceleration and deceleration (BDTarc).
Results
With a similar total number of energy layers, SPArc_seq plans provided a similar nominal plan quality and plan robustness compared to SPArc_orig plans. SPArc_seq significantly reduced the number of ascended ELS by 83% (19 vs.115), 70% (16 vs. 64), 82% (19 vs. 104), 80% (19 vs. 94) and 70% (9 vs. 30), which effectively shortened the BDTfixed by 65% (386 vs. 1091 s), 61% (235 vs. 609 s), 64% (336 vs. 928 s), 48% (787 vs.1521 s) and 25% (384 vs. 511 s) and shortened BDTarc by 54% (522 vs.1128 s), 52% (310 vs.645 s), 53% (443 vs. 951 s), 49% (803 vs.1583 s) and 26% (398 vs. 534 s) in prostate, lung, brain, HNC and breast cancer, respectively.
Conclusions
The SPArc_seq optimization algorithm could effectively reduce the BDT compared to the original SPArc algorithm. The improved efficiency of the SPArc_seq algorithm has the potential to increase patient throughput, thereby reducing the operation cost of proton therapy.
Introduction
Intensity-modulated proton therapy (IMPT), based on the pencil beam scanning (PBS) technique, has become a popular treatment modality in radiation therapy [Citation1,Citation2]. Compared to the traditional passive scattering proton therapy [Citation3,Citation4], IMPT is able to improve the dose conformity at the proximal end and further reduce the dose to the adjacent healthy tissue [Citation3,Citation4]. However, IMPT faces more challenges compared to the passive scattering technique, such as varieties of uncertainties from breathing-induced motion, patient setup and proton range [Citation5–10], large lateral penumbras due to the spot size and scattering [Citation11], as well as the number of beam angles where three to four fields at maximum were normally used in the clinical practice [Citation12–14]. To address these challenges, Ding et al. proposed spot-scanning proton arc therapy (SPArc) as a new planning and treatment platform in 2016 [Citation15].
The SPArc algorithm incorporates a series of iterative spot and energy selections into robust optimization. It generates the energy layers and spot positions with fine sampled control points for a continuous proton arc delivery [Citation15,Citation16]. Previous studies have found that SPArc could improve the dosimetric plan quality in prostate, lung, head neck and brain cancer treatments in comparison with the conventional multi-field IMPT [Citation17–20].
Normally, a SPArc plan contains hundreds of control points via an arc trajectory in which each control point consists of several energy layers. Unlike IMPT, where the energy layers of each treatment field are delivered in the descending order, SPArc has to switch energy layers back and forth in a random order between the control points during the treatment delivery [Citation15,Citation13,Citation21]. Due to the magnetic hysteresis effect, it takes much longer for the proton system to switch the energy layer upward to a higher energy than downward to lower energy [Citation22–26]. In order to mitigate such impact on beam delivery time (BDT) from the prolonged ELS upward, one strategy is to deliver the energy layers sequentially between the control points and minimize the number of ELS in the ascending order following the direction of the gantry rotation. Thus, we presented a new energy layer sequence optimized SPArc algorithm (SPArc_seq) for the proton arc treatment delivery.
Method and materials
Original SPArc optimization algorithm
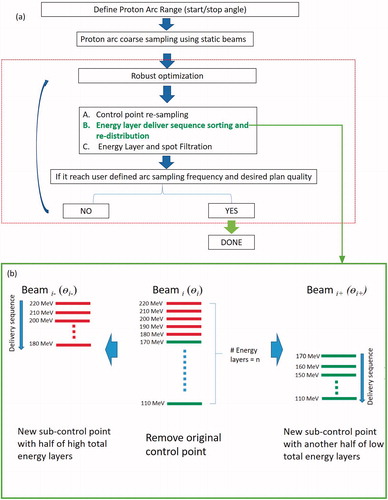
The original SPArc optimization algorithm (SPArc_orig) starts from a coarse sampling frequency using the worst-case scenario robust optimization to achieve a proton arc plan [Citation15]. The algorithm integrated the iterative approaches includes [Citation1] control point re-sampling; [Citation2] control point energy layers re-distribution; [Citation3] energy layers filtration; and [Citation4] energy layers re-sampling and [Citation5] spot number reduction.
SPArc_seq optimization algorithm
In contrast to the SPArc_orig, which distributes the energy layers randomly to the nearby sub-control points, the SPArc_seq algorithm sorts and re-distributes the energy layer in the descending order between these sub-control points following the direction of proton arc delivery and gantry rotation during the iterative optimization approach (). In other words, the higher energy layers were assigned to earlier delivered sub-control point (Beami-), while the lower energy layers were assigned to the following sub-control point (Beami+) (). Such iterative energy layer sorting and re-distribution mechanism can minimize the number of ascending ELS during delivery of the proton arc therapy compared to the SPArc_orig algorithm. Given that the ELS time in the ascending order is longer than the ELS time in the descending order [Citation25], the SPArc_seq algorithm has the potential to shorten BDT significantly in a proton arc plan with hundreds of energy layers.
Figure 1. (a) SPArc algorithm and workflow. (b) The schematic diagram of SPArc_seq algorithm: energy layer sorting and re-distribution split mechanism between the sub-control point. Beami represented the i-th beam in the coarse IMPT plan. The beami− and beami+ were the corresponding two sub-control points generated by splitting Beami.
More details of the energy sorting and redistribution algorithm can be described as following: There were total m control points or beams in the initial plan with a coarse sampling frequency. Let i represent the i-th beam, and the corresponding beam included three elemental characteristics: gantry angle energy layer set Ei, monitor unit (MU) set MUi, where energy layer set Ei consists of total n energy layers, any energy layer was donated as Ei,j, that is
(1)
(1)
in which the energy layers were sorted in the descending order as the index j increase. The corresponding MU set was denoted as MUi as well as any energy included mui,j, which was expressed as
(2)
(2)
where
[] represents integer operation. Instead of assigning energy layer randomly via the original SPArc algorithm, the initial i-th beam was split by
using energy sorting mechanism via SPArc_seq to create two new beams, in which one beam contains the higher energy layers set and the other beam contains lower energy layers set, the corresponding three elemental characteristics of the two sub-control points were donated as
Ei+, MUi+ and
Ei-, MUi−, respectively, where
(3)
(3)
(4)
(4)
This energy sorting and redistribution mechanism were implemented in the iterative inverse optimization () until the angle sampling frequency reached the user-defined parameter, e.g., 2.5 deg per control point. Thus, the new SPArc_seq algorithm could reduce the number of ascended ELS during the proton arc delivery.
Plan quality evaluation
Five representative disease cases, including prostate, lung, brain, HNC and breast cancer, were selected to test the performance of the SPArc_seq and SPArc_orig algorithms. Both planning groups were generated using the same objectives and arc sampling frequency (2.5 deg per control point) in Raystation version 6.02 (RaySearch Laboratories AB, Stockholm, Sweden). The prescription dose, parameters of the robust optimization and beam arrangement are listed in Supplemental Document: Table 1.
The dose-volume histograms (DVHs) of both target volumes and OARs were used to compare the nominal plan quality between SPArc_seq and SPArc_orig planning groups. For plan robustness evaluation, the perturbed doses were generated with 3 or 5 mm isocenter shifts in the anterior–posterior, superior–inferior and right–left directions under the nominal proton beam range and ±3.5% proton beam range uncertainties, corresponding to a total of 21 dose distribution scenarios. All the DVHs of perturbed doses as well as nominal dose were plotted to assess the plan robustness.
Fixed gantry beam delivery and QA measurements
In order to eliminate the current hardware and devices related issues, both SPArc_seq and SPArc_orig plans were delivered at a fixed gantry angle of 90° using an IBA Proteus®One proton system [Citation27]. This method is considered as the best-case-scenarios because fixed gantry treatment delivery does not take into account of the gantry rotation and mechanical limitations. To validate if the clinical proton system can deliver the radiation accurately with a treatment plan consisting of hundreds of energy layers and numerous spots with small MUs which is close to the machine limitation (0.02 MU per spot as the minimum threshold in IBA ProteusONE). The plan quality assurance (QA) measurements were performed using MatriXXONETM (IBA Dosimetry, Schwarzenbruck, Germany), a 2D ionization chamber array, with 3 cm solid water buildup. The 2D gamma index (GI) using 3%, and 3 mm criteria were used to evaluate the agreement between measured and calculated 2D dose distributions [Citation28]. The proton machine’s log files during the delivery were analyzed to evaluate the total BDT from the fixed gantry delivery.
Dynamic proton arc scanning controller simulation
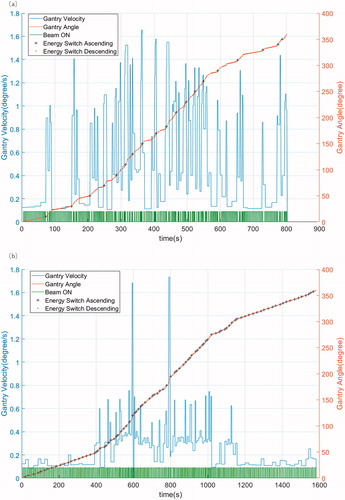
The total BDTarc might be different from BDTfixed, when gantry rotation and mechanical limitation are considered. As the current clinical proton system was not designed for the dynamic arc therapy delivery initially, it requires several hardware upgrades to deliver the SPArc treatment in a smooth and safe manner. In this study, we designed an in-house dynamic SPArc controller simulating the rotational gantry treatment delivery. The SPArc controller takes into account of the dynamic gantry rotation limitation by calculating the speed of the gantry, acceleration and deceleration connecting each individual control point. The mechanical parameters of the IBA ProteusONE system were used in this SPArc controller such as maximum gantry speed of 6 deg/s and maximum acceleration/deacceleration of 0.6 deg/s2. Delivery tolerance window is set at 2.5 deg, or in other word, ±1.25 deg.
Results
Plan parameters and dosimetric plan quality evaluation
Both SPArc_seq and SPArc_orig plans consisted of similar plan parameters, including the total number of total energy layers and total MUs (). For example, in HNC case, SPArc_seq has a total of 222 energy layers, while SPArc_orig plan has a total of 219 energy layers. There was less than 2% difference in total MU between SAPrc_seq and SPArc_orig plans. Moreover, MU weighting through energy layers distributions were very similar as well (Supplemental Document: Figure 1). Overall, both SPArc_seq and SPArc_orig plans achieved a similar dose distribution in the representative cases ().
Figure 2. A representative CT slice of an HNC case with a transversal view of dose distribution. (a) SPArc_seq plan; (b) SPArc_orig plan and (c) dose difference between SPArc_seq and SPArc_orig; (d) the corresponding DVHs (SPArc_seq: solid line, SPArc_orig: dash line). (e) The DVHs of nominal position (solid line) and 20 scenarios of worst-case scenarios (dashed line) for CTVs and OARs. The similar perturbed DVH band indicates a similar plan robustness.
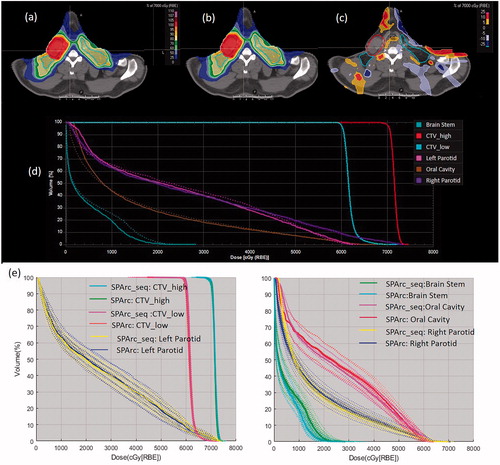
Table 1. The plan parameters for SPArc_seq and SPArc_orig plan.
Plan robustness evaluation
The perturbed dose distributions were generated under 21 worst-case-scenarios for both SPArc_seq and SPArc_orig. The corresponding DVHs of perturbed doses and the nominal dose of CTV_high, CTV_low, brain stem, left parotid, right parotid and oral cavity are displayed in , respectively. The result suggested that SPArc_seq could achieve similar plan robustness as the SPArc_orig in all disease sites tested.
QA measurements and analysis of the beam delivery time at the fixed gantry angle
The QA results showed that absolute dose difference between plan dose and measured dose was within 2%, and the 2D GI passing rates were all above 97 using 3% and 3 mm criteria in both SPArc_seq and SPArc_orig planning groups (Supplemental Document: Table 2). The result indicated that either SPArc_seq or SPArc_orig plans with numerous small weighting MU spots and energy layers were able to be delivered accurately and met the clinical requirement using an existing clinical proton beam therapy system ().
Figure 3. An example of HNC case for (a) MU per Spot vs. Spot Number; (b) A comparison between SPArc_seq and SPArc_orig in the energy layer delivery sequence. CW: clockwise. (c) The total number of energy layers vs. cost function value; and BDT tested in the two tested plans with 222 and 120 total energy layers.
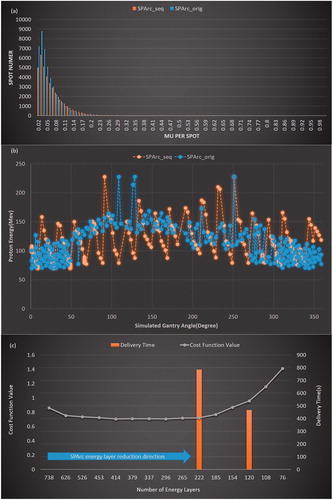
The proton machine log files recorded the beam delivery on a 1 ms sampling frequency. The analysis of the machine log files showed that the average ELS time was 5.71 ± 0.16 s (ascended ELS) and 1.23 ± 0.19 s (descended ELS). SPArc_seq algorithm significantly reduced the number of ascended ELS by 83% (19 vs. 115) , 70% (16 vs. 64), 82% (19 vs. 104), 80% (19 vs. 94) and 70% (9 vs.30), in prostate, lung, brain, HNC and breast cancer cases, respectively, compared to SPArc_orig (). As a result, the SPArc_seq plan successfully shortened the BDTfixed by 65% (386 vs. 1091 s), 61% (235 vs. 609 s), 64% (336 vs. 928 s), 48% (787 vs. 1521 s) and 25% (384 vs. 511 s), among these cases, respectively. shows an example of a comparison between SPArc_seq and SPArc_orig plans in the energy layer delivery sequence in the HNC case.
Dynamic SPArc controller simulation
The speed of the gantry rotation, proton beam delivery status with ‘ON’ or ‘OFF’, energy switch ascending or descending, accumulated gantry angle and BDTarc were calculated through the SPArc controller. The example of the HNC case is displayed in . The SPArc_seq plan successfully shortened the BDTarc by 54% (522 vs.1128 s), 52% (310 vs.645 s), 53% (443 vs.951 s), 49% (803 vs.1583 s) and 26% (398 vs. 534 s) in prostate, lung, brain, HNC and breast cancer cases, respectively ().
Discussion
In this study, we proposed a novel and improved SPArc algorithm to optimize the energy layer delivery sequence in proton arc therapy by implementing an energy layer sorting and re-distribution mechanism during the control point re-sampling process. The new algorithm SPArc_seq was tested on the five different disease sites and evaluated using two methods: fixed gantry delivery and dynamic SPArc controller simulation. The result demonstrates that the SPArc_seq optimization algorithm successfully improves the proton arc delivery efficiency and maintains similar plan quality and robustness, compared to the SPArc_orig algorithm.
The BDT of conventional IMPT is primarily determined by the proton beam irradiation time and ELS time, however, ELS time generally dominates the total BDT [Citation13,Citation29] and SPArc follows the same principles as it can be considered an advance IMPT plan with hundreds of beam angles and energy layers. With the implementation of the new SPArc_seq algorithm, the component of ELS time could be reduced in both fixed gantry delivery mode and dynamic SPArc controller simulation (). For disease sites with large target volumes, such as bilateral HNC and breast cancer cases, proton beam irradiation time starts dominating the BDTfixed as it requires much more proton spots to cover the entire volume. However, the component of ELS time still dominated the BDTfixed in the disease sites where the target volume is relatively small, such as prostate, brain and lung cancer cases. With the cost to the plan quality, there might still be room to shorten the BDTfixed by further reducing the total number of energy layers. We tested the HNC case and plotted the plan cost function value as a function of the total number of energy layers (). The cost function value decreased at the beginning but increased rapidly when the number of energy layers was less than 200 (). The experiment found that the BDTfixed could be further reduced to 487 s with a SPArc_seq plan of 120 energy layers compared to 786 s with a SPArc_seq plan of 216 energy layers. Such improvement in the treatment delivery efficiency increased the cost function value by 33% from 0.7204 to 0.9567. It is noteworthy to address the balance between the treatment delivery efficiency and the plan quality in the proton arc plan optimization in future studies.
Lastly, SPArc, as new prototype proton therapy technique, is still under rapid research and development. To ensure a safe, smooth and accurate treatment technique for clinical implementation, the architecture of the proton system might need to be modified so it could synchronize the gantry rotation with beam delivery system and patient position system. Some new hardware and devices upgrades may be required, such as ion chamber and electrometer to reduce the impact from the noise during the gantry rotation. Once the engineering challenges were solved, there will be more information and new updates related to delivery accuracy and efficiency of the dynamic SPArc therapy.
Conclusions
The SPArc_seq optimization algorithm was able to not only achieve a similar plan quality and robustness but also improve the proton arc delivery efficiency compared to the original SPArc algorithm. Such improvement in the proton arc delivery efficiency could pave the road for future clinical implementations and has the potential to increase the patient throughput of a proton therapy center.
Supplemental Material
Download MS Word (12.5 KB)Supplemental Material
Download MS Excel (9.5 KB)Supplemental Material
Download JPEG Image (200.7 KB)Acknowledgments
The authors appreciate the valuable input from Guillaume Ganssens and Olivier De Wilde, from IBA, on the proton gantry mechanical limitation.
Disclosure statement
Xuanfeng Ding, Xiaoqiang Li and Di Yan have a patent related to the Particle Arc Therapy (WO2017156419).
Additional information
Funding
References
- Lomax A. Intensity modulation methods for proton radiotherapy. Phys Med Biol. 1999;44(1):185–205.
- Lomax AJ, Boehringer T, Coray A, et al. Intensity modulated proton therapy: a clinical example. Med Phys. 2001;28(3):317–324.
- Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: a virtual clinical study. Int J Radiat Oncol. 2010;77(2):357–366.
- Chang JY, Li H, Zhu XR, et al. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2014;90(4):809–818.
- Liu W, Li Y, Li X, et al. Influence of robust optimization in intensity-modulated proton therapy with different dose delivery techniques. Med Phys. 2012;39(6):3089–3101.
- Liu W, Frank SJ, Li X, et al. Effectiveness of robust optimization in intensity-modulated proton therapy planning for head and neck cancers: robust optimization for IMPT for H&N cancer. Med Phys. 2013;40(5):051711.
- Langen K, Zhu M. Concepts of PTV and robustness in passively scattered and pencil beam scanning proton therapy. Semin Radiat Oncol. 2018;28(3):248–255.
- Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions. Phys Med Biol. 2008;53(4):1043–1056.
- Unkelbach J, Bortfeld T, Martin BC, et al. Reducing the sensitivity of IMPT treatment plans to setup errors and range uncertainties via probabilistic treatment planning: optimizing IMPT plans under uncertainty. Med Phys. 2008;36(1):149–163.
- Fredriksson A, Forsgren A, Hårdemark B. Minimax optimization for handling range and setup uncertainties in proton therapy: minimax optimization for handling uncertainties in proton therapy. Med Phys. 2011;38(3):1672–1684.
- Moignier A, Gelover E, Wang D, et al. Theoretical benefits of dynamic collimation in pencil beam scanning proton therapy for brain tumors: dosimetric and radiobiological metrics. Int J Radiat Oncol Biol Phys. 2016;95(1):171–180.
- Albertini F, Gaignat S, Bosshardt M, et al. Planning and optimizing treatment plans for actively scanned proton therapy. In: Biomedical mathematics: promising directions in imaging, therapy planning and inverse problems. Medical Physics Publishing; 2010. Chapter 1; p. 1–18.
- van de Water S, Kooy HM, Heijmen BJM, et al. Shortening delivery times of intensity modulated proton therapy by reducing proton energy layers during treatment plan optimization. Int J Radiat Oncol. 2015;92(2):460–468.
- Ding X, Li X, Qin A, et al. Redefine the role of range shifter in treating bilateral head and neck cancer in the era of intensity modulated proton therapy. J Appl Clin Med Phys. 2018;19:749–755.
- Ding X, Li X, Zhang JM, et al. Spot-scanning proton arc (SPArc) therapy: the first robust and delivery-efficient spot-scanning proton arc therapy. Int J Radiat Oncol Biol Phys. 2016;96(5):1107–1116.
- Li X, Liu G, Janssens G, et al. The first prototype of spot-scanning proton arc treatment delivery. Radiother Oncol. 2019;137:130–136.
- Ding X, Li X, Qin A, et al. Have we reached proton beam therapy dosimetric limitations? – A novel robust, delivery-efficient and continuous spot-scanning proton arc (SPArc) therapy is to improve the dosimetric outcome in treating prostate cancer. Acta Oncol. 2018;57(3):435–437.
- Li X, Kabolizadeh P, Yan D, et al. Improve dosimetric outcome in stage III non-small-cell lung cancer treatment using spot-scanning proton arc (SPArc) therapy. Radiat Oncol. 2018;13(1):35.
- Ding X, Zhou J, Li X, et al. Improving dosimetric outcome for hippocampus and cochlea sparing whole brain radiotherapy using spot-scanning proton arc therapy. Acta Oncol. 2019;58(4):483–490.
- Liu G, Li X, Qin A, et al. Improve the dosimetric outcome in bilateral head and neck cancer (HNC) treatment using spot-scanning proton arc (SPArc) therapy: a feasibility study. Radiat Oncol. 2020;15(1):21.
- Schippers JM, Lomax AJ. Emerging technologies in proton therapy. Acta Oncol. 2011;50(6):838–850.
- Han W, Liu X, Qin B, et al. Design considerations of a fast kicker system applied in a proton therapy beamline. Nucl Instrum Methods Phys Res A. 2019;940:199–205.
- Tani N, Adachi T, Someya H, et al. Eddy current effect of magnets for J-PARC 3-GeV synchrotron. IEEE Trans Appl Supercond. 2004;14(2):421–424.
- Liu X, Qin B, Liu K, et al. Eddy current analysis and optimization of fast scanning magnet for a proton therapy system. Nucl Instrum Methods Phys Res Sect Accel Spectromet Detect Assoc Equip. 2017;862:1–7.
- Farr JB, Flanz JB, Gerbershagen A, et al. New horizons in particle therapy systems. Med Phys. 2018;45(11):e953–e983.
- Schippers JM, Seidel M. Operational and design aspects of accelerators for medical applications. Phys Rev Spec Top Accel Beams. 2015;18:034801.
- Van de Walle J, Abs M, Conjat M, et al. The S2C2: from source to extraction. In: Proceedings of the 21st International Conference on Cyclotrons and Their Applications (Cyclotrons’16); 2016; Zurich, Switzerland. p. 285–289. Available from: https://accelconf.web.cern.ch/cyclotrons2016/papers/thb01.pdf
- Low DA, Harms WB, Mutic S, et al. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25(5):656–661.
- Shen J, Tryggestad E, Younkin JE, et al. Technical note: using experimentally determined proton spot scanning timing parameters to accurately model beam delivery time. Med Phys. 2017;44(10):5081–5088.