Introduction
Evidence-based consensus guidelines have established the indications for radiation in the postoperative setting following radical prostatectomy for prostate cancer [Citation1]. Adjuvant radiotherapy has shown benefits in biochemical relapse-free- and overall survival in large cooperative group randomized clinical trials despite mild to moderate increased toxicity [Citation2–5] For salvage radiotherapy, large retrospective series have similarly shown benefits in biochemical relapse-free survival and overall survival [Citation6,Citation7]. Level one evidence continues to evolve suggesting a benefit to concurrent androgen deprivation therapy (ADT) [Citation8,Citation9] and elective pelvic nodal irradiation [Citation10] in the salvage setting. Despite these oncologic benefits, concern regarding the treatment toxicities of radiotherapy may hinder timely referral for consideration of radiotherapy in these prostate cancer patients already suffering from urinary and sexual dysfunction following their radical prostatectomy [Citation11].
Proton therapy (PT) has recently been explored to reduce radiation dose exposure in the post-prostatectomy setting, increasingly facilitated by the advancement of scanning beam PT, which allows for the treatment of more complex and irregular targets such as the prostate bed, as well as the development of onboard image guided techniques for PT providing real-time assessment of daily targeting. Initial assessments have demonstrated its feasibility, reductions in the low and intermediate dose exposure of the adjacent bladder and rectum compared to intensity modulated photon radiotherapy (IMRT), and a favorable toxicity profile comparable to IMRT [Citation12,Citation13]. While these dosimetric advantages and initial toxicity profiles have been recently reported, and prospective, nonrandomized [Citation14,Citation15] and randomized [Citation16] clinical trials are ongoing, the initial oncologic outcomes with post-prostatectomy PT have not yet been reported to our knowledge. The purpose of our study was to report the initial oncologic outcomes for the first 100 patients undergoing post-prostatectomy PT at a single institution and to assess associations with biochemical failure-free survival (BFFS).
Methods and materials
We conducted an institutional review board–approved retrospective analysis of the first 100 consecutive patients undergoing post-prostatectomy PT at a single institution. Patients were treated from 2010 to 2016. Patients had histologically-confirmed, non-metastatic prostate adenocarcinoma status post radical prostatectomy and were generally evaluated by complete history, physical examination, bone scintigraphy, and computed tomography (CT) of the abdomen and pelvis and/or pelvic magnetic resonance imaging (MRI) ± endorectal coil. Concurrent elective pelvic nodal irradiation, classically termed whole pelvis, and/or concurrent ADT, consisting of luteinizing hormone-releasing hormone (LHRH) analog administration for a median of 7 months (range 4–26 months) initiated prior to PT, were at the physician’s discretion and offered primarily to patients with adverse risk features (PSA >10 ng/mL, T3a/T3b, and/or Gleason ≥7). Patients were monitored weekly during treatment and generally seen 3 months after PT completion and every 6 months thereafter. Initial toxicity outcomes with a median follow up of 25 months have been previously reported for this same cohort [Citation12]. Additional toxicity outcomes with a median follow up of 46 months for the subset of patients treated to the prostate bed only have also been reported in a matched comparison to patients undergoing IMRT [Citation13].
Radiotherapy planning and delivery including daily image guided RT with kV imaging were as previously described [Citation12]. The prostate bed ± whole pelvis were delineated as the clinical target volume(s) (CTV) according to consensus guidelines [Citation17,Citation18]. PT and IMRT treatment plans were generated using Eclipse Treatment Planning (Software version 10; Varian, Palo Alto, CA). To account for proton beam range uncertainty, a margin in the beam direction of 3.5% of the beam range was applied to correct for uncertainty in conversion from Hounsfield units to proton stopping power with an additional 1 mm margin to correct for beam calibration uncertainty. For treatment plan optimization purposes for pencil beam scanning, a pencil beam scanning target volume (PBSTV) was created to correct upfront for these uncertainties [Citation19]. The prostate bed planning target volume (PT) was created as a 10-mm uniform expansion, except 6-mm posteriorly, from the CTV, with the expansion based on lateral uncertainty alone as recommended by ICRU 78 [Citation20], and used for recording and reporting purposes. For whole pelvis PT, a 5–8 mm uniform expansion was similarly utilized. Minimum CTV and PT coverage was D98 > 98% and D95 > 95%, respectively. All nominal plans were normalized to the mean dose of the CTV for comparison purposes. Prescription doses to the whole pelvis and prostate bed CTVs were 50.4 and 66.6–70.2 Gy (RBE) in 1.8 Gy (RBE) fractions; eight patients received an additional prostate bed boost for gross recurrence to 75.6 Gy (RBE), for a constant RBE of 1.1. When target coverage and organs at risk dose constraints could not achieve predefined institutional dose constraints with PT alone due to anatomical or technical modality challenges, combined IMRT plans were used to achieve dose constraints; this included 17/20 whole pelvis patients and 14/80 patients treated to the prostate bed only.
Statistical analysis
Baseline patient characteristics and the following failure endpoints were retrospectively assessed from the start of radiation and descriptively reported: (1) biochemical failure (defined as two consecutive rises above the nadir, obvious clinical progression, or initiation of salvage therapy such as ADT), (2) first site of clinical failure – local, regional, and/or distant metastasis, and (3) overall survival were recorded. BFFS, distant metastasis free-, and overall survival curves were estimated by the Kaplan-Meier product-limit method; patients without failure were censored at the last clinical encounter. The Cox proportion hazards model was used to assess uni- and multivariable association with biochemical failure and the clinical and treatment characteristics noted in the Supplemental Table 1; variables with p values of <.1 in the univariable analysis were included in the multivariable model. A p value of <.05 was considered statistically significant. The only parameter with greater than 10% missing data was pre-operative PSA. Patients with and without missing data did not differ in observed variables. Statistical analyses were conducted using Stata version 14 software (StataCorp, College Station, TX).
Results
The cohort characteristics are summarized in . Median age and months after surgery were respectively 64 years (range 42–77) and 25 (range 5–216). PT received was 70.2 Gy (RBE) (89%), in the salvage setting (93%), prostate bed only (80%), pencil beam scanning technique (86%), with IMRT (31%), and with ADT (34%). Median follow-up was 55 months (range 16–80).
Table 1. Post-prostatectomy proton therapy cohort baseline demographic, clinical, and treatment characteristics.
In terms of the crude failure rates, biochemical failure was noted in 39 patients (39%). Median time to biochemical failure was 23 months (5–69). For patients with biochemical failure, local failure within the prostate bed was noted in 1 (1%) patient 30 months from salvage PT with a solitary recurrence noted on fluciclovine PET/CT in the left prostatectomy bed without evidence of distant radiotracer-avid metastatic disease.
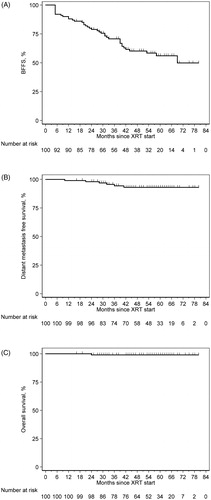
Regional pelvic nodal failure was noted in four patients (4%) – all treated to the prostate bed only – at median 32 months (10–38), two of whom also had distant metastasis. Distant metastasis occurred in six patients (6%) at median 30 months (10–41), five with bony and 1with lung involvement. There was 1 death at 24 months, unrelated to prostate cancer. shows the Kaplan Meier estimates for BFFS, distant metastasis free-, and overall- survival for the entire cohort, which at 5 years were 56% (95% CI: 44–66%), 93% (95% CI: 85–97%), and 99% (95% CI: 93–99%), respectively.
Figure 1. Kaplan-Meier survival estimates for (A) biochemical failure-free survival, (B) distant metastasis free survival, and (C) overall survival for post-prostatectomy proton therapy.
Supplemental Table 1 shows the results of the univariable analysis with all variables included. The only clinical factors with univariable association with BFFS of p < .1 were Gleason score >7 (HR 3.080, 95% CI 1.597, 5.939; p = .001) and whole pelvis RT (HR 0.378, 95% CI 0.134, 1.065, p = .066) (Supplemental Table 2). On multivariable analysis, both factors were significant: Gleason score >7 vs. 7 (HR 3.530, 95% CI 1.824, 6.833; p < .001) and whole pelvis RT vs. prostate bed field only (HR 0.238, 95% CI 0.083, 0.684; p = .008). Supplemental Figure 1 shows the Kaplan Meier estimates for BFFS stratified by Gleason score and whole pelvis RT. Five year BFFS for Gleason <7, 7, and >7 were 5 years were 85% (95% CI: 51–96%), 65% (95% CI: 50–76%), and 18% (95% CI: 5–41%), respectively, and for whole pelvis (79%; 95% CI: 53–91%) vs. prostate bed (50%; 95% CI: 37–62%).
Discussion
In this study, we report for the first time, the oncologic outcomes of post-prostatectomy PT and demonstrate that PT is feasible, with comparable clinical outcomes to historical photon outcomes. Gleason score greater than 7 and whole pelvis RT were the only clinical or treatment-related factors associated with BFFS on multivariable analysis.
BFFS compared favorably in this proton cohort to previous oncologic outcomes for photon therapy (Supplemental Table 2). Nonetheless, direct comparisons to existing trials are difficult given the lack of a consistent failure definition across adjuvant and salvage trials. In the current study, biochemical failure was defined as two consecutive rises above the nadir, obvious clinical progression, or initiation of salvage therapy such as ADT, and BFFS was 56%. In a combined, multi-institutional retrospective review of 2460 patients treated with salvage radiation at 10 tertiary care centers between 1987 and 2013, biochemical failure was defined as a PSA of 0.2 above the post-treatment nadir (with a confirmatory value) or initiation of salvage ADT after completion of RT; 5 year freedom from biochemical progression was similarly 56% [Citation21]. Although we only found an association with Gleason score and whole pelvis field with BFFS in this initial proton cohort, in the aforementioned study, multiple clinical and treatment-related parameters were associated with biochemical progression, such as pre-RT PSA, Gleason score, and the use of ADT.
Since the initiation of treatment in this study cohort, increasing evidence suggests a benefit to ADT in the salvage setting [Citation8,Citation22]. In GETUG-AFU 16, a randomized phase III trial of salvage radiation with or without ADT, biochemical failure was less stringently defined as a PSA of 0.5 ng/mL above the nadir (confirmed by a second PSA measurement) or clinical progression. At 5 years, 62% of patients receiving radiation alone – comparable to the 56% noted in the current proton cohort – and 80% of patients receiving radiation and ADT were free from biochemical progression. The RTOG 9601 similarly showed a benefit to concurrent ADT in the salvage setting, and patients with Gleason score less than or equal to 7, showed improved clinical outcomes including 12 year overall survival, compared to those with Gleason greater than 7.
Preliminary results from RTOG 0534 have been reported in abstract form only. In this randomized phase III trial of salvage radiation to the prostate bed vs prostate bed and ADT vs whole pelvis and prostate bed and ADT, biochemical failure was defined as maintenance of a PSA less than the nadir + 2 ng/mL (and absence of clinical failure and absence of death from any cause for 5 years). At 5 years, freedom from progression was noted to be 72%, 83%, and 89% in those arms respectively, favoring the whole pelvis field. In the current proton cohort, 5 year BFFS for patients treated to the whole pelvis was 79% and only 50% if treated to the prostate bed only. Of note, a more stringent biochemical failure definition – PSA ≥ 0.4 ng/mL and rising at 5 years after randomization – is noted as a secondary outcome and not yet reported.
The heterogeneity of this cohort composed of patients treated with or without ADT and/or elective pelvic nodal irradiation and/or IMRT represents a potential limitation of this study, however it was felt to represent a real-world cohort of post-prostatectomy cases presenting for RT and eligible PT from the outset. Ultimately, nearly one-third of patients required mixed use of proton and IMRT modalities to achieve predefined dose constraints and what was ultimately deemed the best possible radiotherapy plan. The retrospective nature of the study and the limited number of patients and follow up represent additional limitations. Distant metastases and mortality will likely increase, and continued follow-up could reveal associations with other parameters. Nonetheless, we felt that an initial report was merited since the oncologic outcomes of post-prostatectomy PT have not yet been reported and PT access increases worldwide.
In conclusion, post-prostatectomy PT for prostate cancer is feasible with comparable oncologic outcomes to photon therapy through early follow up in the first 100 patients undergoing adjuvant or salvage therapy at a single institution.
Supplemental Material
Download MS Word (61.3 KB)Supplemental Material
Download MS Word (35 KB)Disclosure statement
There are no actual or potential conflicts of interest to disclose for any of the authors.
Additional information
Funding
References
- Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol. 2013;190(2):441–449.
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018–2027.
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–962.
- Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66(2):243–250.
- Hackman G, Taari K, Tammela TL, et al. Randomised trial of adjuvant radiotherapy following radical prostatectomy versus radical prostatectomy alone in prostate cancer patients with positive margins or extracapsular extension. Eur Urol. 2019;76(5):586–595.
- Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. JCO. 2007;25(15):2035–2041.
- Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299(23):2760.
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417–428.
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17(6):747–756.
- Pollack A, Karrison TG, Balogh AG, Jr, et al. Short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiotherapy: the NRG Oncology/RTOG 0534 SPPORT Trial. Int J Radiat Oncol Biol Phys. 2018;102(5):1605.
- Deville C, Vapiwala N, Hwang WT, et al. Comparative toxicity and dosimetric profile of whole-pelvis versus prostate bed-only intensity-modulated radiation therapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2012;82(4):1389–1396.
- Deville C, Jr, Jain A, Hwang WT, et al. Initial report of the genitourinary and gastrointestinal toxicity of post-prostatectomy proton therapy for prostate cancer patients undergoing adjuvant or salvage radiotherapy. Acta Oncol. 2018;57(11):1506–1514.
- Santos PMG, Barsky AR, Hwang WT, et al. Comparative toxicity outcomes of proton beam therapy versus intensity-modulated radiotherapy for prostate cancer in the post-operative setting. Cancer. 2019;125(23):4278–4293.
- https://clinicaltrials.gov/ct2/show/NCT00969111. [cited 2019 Oct 27].
- https://clinicaltrials.gov/ct2/show/NCT03570827. [cited 2019 Oct 27].
- Koerber SA, Katayama S, Sander A, et al. Prostate bed irradiation with alternative radio-oncological approaches (PAROS) - a prospective, multicenter and randomized phase III trial. Radiat Oncol. 2019;14(1):122.
- Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76(2):361–368.
- Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74(2):383–387.
- Kirk ML, Tang S, Zhai H, et al. Comparison of prostate proton treatment planning technique, interfraction robustness, and analysis of single-field treatment feasibility. Pract Radiat Oncol. 2015;5(2):99–105.
- International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting proton-beam therapy (ICRU Report 78). Oxford: Oxford University Press; 2007. (vol. 7, no. 2). Chapter 6: Treatment Planning; p. 96–99.
- Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. JCO. 2016;34(30):3648–3654.
- Carrie C, Magné N, Burban-Provost P, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019.
