Abstract
Background
Colorectal cancer is the fourth leading cause of cancer-associated death in the world. The 5-year local recurrence rates in patients undergoing multimodality therapy are approximately 5–10%. The standard approach to treat locally recurrent rectal is re-irradiation followed by surgical resection. Recent reports have suggested that the treatment outcomes with carbon ion radiation therapy (CIRT) in recurrent rectal cancer are promising and have superior results compared to photon therapy. Hence, we performed a systematic review to evaluate the patterns of care and treatment outcomes of recurrent rectal cancer patients treated with CIRT.
Methodology
We performed a systematic search to identify the articles that reported on CIRT use in recurrent rectal cancer.
Results
Systematic search of PubMed and Cochrane Central resulted in 98 abstracts. Eight studies fulfilled the predefined inclusion criteria. Among eight studies, one study is a prospective phase I/II study done in Japan; three prospective studies are ongoing (PANDORA-01 trial, HIMAT1351trial, and a phase II study of reirradiation for prior CIRT), and five studies are institutional reports on role of CIRT. These studies were predominantly reported from Japan and Germany. All reports except one were performed in patients who have not received prior radiation. The most commonly utilized treatment prescription was 73.4 Gy (RBE) in 16 fractions over 4 weeks in patients without any prior history of radiation and 36 Gy in 12 fractions over 3 weeks at 3 Gy per fraction in patients with prior photon radiation to the pelvis. There is one ongoing trial assessing the role of carbon ion re-irradiation in patients who had prior CIRT for rectal cancer.
Conclusion
CIRT holds immense promise in improving outcomes in locally recurrent rectal cancer. There is a need for more multi-institutional prospective clinical trials to assess the role of CIRT.
Introduction
Cancer of the colorectum is one of the most commonly diagnosed malignancies worldwide, accounting for a global burden of 1.4 million new cases per year and constituting the fourth leading cause of cancer-associated death [Citation1]. The rate of local recurrence in locally advanced rectal cancer patients treated with a combined modality approach continues to be around 5–10% [Citation2–4]. The incidence of locally recurrent rectal cancer (LRRC) has shown a steady decline with the evolution of neoadjuvant radiation or chemoradiation. An alternative approach, the watch and wait approach, in patients who achieve a complete clinical response after neoadjuvant therapy of rectal cancer may herald higher local recurrences to the tune of 25% in patients who are not offered surgery as part of the treatment paradigm [Citation5].
In the subset of the patients who develop a recurrence, the outcomes are very poor with a 3-year survival rate of <30%. This is largely due to the complex anatomical location of these recurrences in relatively inaccessible and heavily pretreated tissues. Dense radiation-induced fibrosis and altered lymphovascular planes that complicate surgery following primary radiation therapy, and fibrosis and associated hypoxia also confer relative radioresistance to these recurrent tumors [Citation6]. Multiple institutional studies have shown that a combination of re-irradiation with photon-based radiotherapy followed by surgery offers the best oncological outcomes with 3-year overall survival (OS) rates of 50% [Citation7]. Multiple reports have shown that re-irradiation of LRRC tends to enhance R0 resection and palliation of symptoms. Strategies to perform re-irradiation have included different techniques such as 3-dimensional conformal radiation therapy (3 D-CRT), intensity-modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT) and/or intra-operative radiation therapy (IORT). Additionally, different fractionation schedules, such as once-daily and twice-daily, in conjunction with other modalities, such as chemotherapy and hyperthermia, have been investigated. Currently, there is interest in investigating particle therapy, including proton and carbon ion radiotherapy, as novel radiation modalities [Citation8].
One of these novel modalities, carbon ion radiotherapy (CIRT), has gained interest in recent years due to several potential benefits over proton and photon irradiation. Carbon-ion beams have a unique depth dose distribution profile characterized by the Bragg peak where little dose is deposited at the entrance of the beam. Most of the energy is deposited as particles slow down at a characteristic depth, so that that the entrance dose is low, the dose at tumor depths is very high, and there is practically no exit dose. Unlike protons though, CIRT incurs a higher probability of a fragmentation tail at the end of the Bragg peak that could increase the dose to tissues immediately distal to the tumor. Carbon ion beams have narrow lateral penumbras, higher linear energy transfer, and are less susceptible to differences in radiation sensitivity as a function of cellular oxygenation than photons or protons [Citation9–11]. The proposed rationale for improved outcomes with CIRT was the enhanced biologically equivalent dose (BED) in excess of 100 Gy that was deliverable with a 70.4 or 73.6 Gy RBE delivered in 16 fractions. There have been multiple reports especially from Japan and Germany regarding the utility of CIRT in rectal cancer. This systematic review seeks to collate the available literature on the role of CIRT in rectal cancer and benchmark its potential clinical implications.
Material and methods
Literature search
The PubMed (National Institutes of Health), and Cochrane Central (Cochrane collaboration) databases were searched with the key words – rectal cancer and carbon ion. The MESH terms used have been attached in the supplementary file. The search spanned the duration from the inception of each database up to 12 November 2019. The search did not have a language filter. BP and PG independently searched the databases and conference proceedings with the application of search terms for the relevant articles and any disagreements were resolved by mutual discussion. SK was responsible for resolving any disagreement in the inclusion of studies.
Eligibility criteria for articles
The following eligibility criteria were used for inclusion and exclusion of articles.
Inclusion criteria: (i) Any prospective/retrospective clinical study (ii) Study should have reported clinical outcomes for rectal cancer, and (iii) Patients must have received CIRT.
Exclusion criteria: (i) Dosimetric studies without any report on patient outcomes, ii) Pre-clinical studies, and case report or case series,
Article review
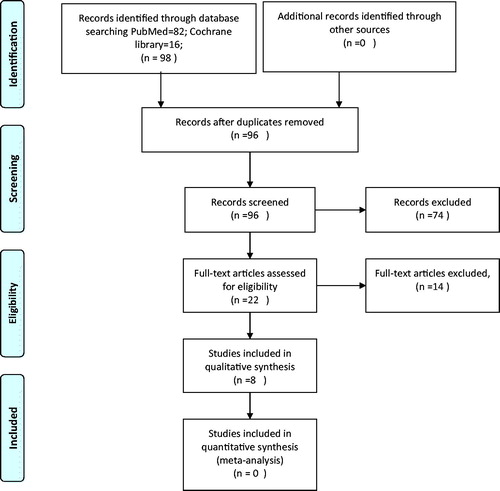
A systematic approach was followed by authors (BP, PG) for reviewing the eligibility of articles. The articles from the initial search of the electronic databases were imported into reference manager software. An independent review of the abstracts and full paper articles was done by BP and PG. The duplicates were removed and the titles of articles were evaluated. Abstracts found to be relevant to the topic of interest were shortlisted. Then the full-length paper of the shortlisted articles was assessed for the eligibility criteria. The articles that fulfilled the inclusion criteria were shortlisted for the final systematic review. The included study references were cross-searched for additional studies. The search strategy has been depicted in the PRISMA flow diagram ().
Results
The systematic search of PubMed and Cochrane Central resulted in 98 abstracts. After removal of duplicates, 96 abstracts were assessed. Twenty-two articles were found to be relevant to the research question. The full-length articles of 22 studies were assessed and 8 studies fulfilled the predefined inclusion criteria. and summarize the studies that are included in this systematic review. Among eight studies, one study is a prospective phase I/II study done in Japan; three prospective studies are ongoing (PANDORA-01 trial, HIMAT1351trial and a phase II study of reirradiation for prior CIRT), and five studies are institutional reports.
Table 1. Summary of clinical studies that have reported on outcomes of carbon-ion radiation therapy in locally recurrent rectal cancer.
Table 2. Summary of ongoing prospective clinical studies of carbon-ion radiation therapy in recurrent rectal cancer.
LRRC without prior radiation
Currently, there are 13 CIRT centers in 5 countries, with the largest series regarding rectal cancer published by J-CROS, a consortium of all the five carbon ion centers currently operational in Japan. The study reported on 224 patients treated with CIRT between 2003 and 2014 in Japan who had a local pelvic recurrence after surgery alone for primary rectal cancer. The patients previously underwent pelvic radiation to a dose of 50 Gy. The most common fractionation schedule was 73.6 Gy (RBE) in 16 fractions (220 patients) while 4 patients received 70.4 Gy (RBE) in 16 fractions. Radiation was given 4 days a week over 4 weeks. Exact beam arrangement and further dosimetry details were not available. The 5-year LC rate was 88% and the 5-year OS rate was 51%. Grade 3 acute toxicity was reported in three patients and grade 3 late toxicities were observed in 12 patients. Details regarding additional adjuvant management, such as chemotherapy or additional surgery, were not available [Citation6,Citation12].
GUNMA 0801 is a prospective observational study performed between 2011 and 2017 assessing CIRT to a dose of 73.6 Gy (RBE) in 16 fractions over 4 weeks in 28 patients with pelvic recurrence of rectal cancer and no prior history of radiation. Passive scattering technique with energies of 290, 380 and 400 MeV/u was used based on the depth of the tumors. No patient underwent surgery after CIRT; 24 patients were not amenable for surgery (17 had sidewall lesions, 7 had presacral lesions) and the remaining 4 refused surgery. The 3-year OS, LC, and progression-free survival (PFS) rates were 92%, 86% and 31%, respectively. Two patients developed late grade 3 pelvic infections [Citation13].
Yamada et al. [Citation14] conducted a stepwise phase I dose escalation and a phase II study in 180 patients with LRRC. In the initial phase, I dose escalation study the dose was escalated stepwise from 67.2 Gy (RBE) to 73.6 Gy (RBE) and 73.6 Gy (RBE) was defined to be the dose for advancement to the phase II study. The patients who were included in the phase II study did not have prior radiation and did not undergo surgery post-resection. The relapse locations where CIRT were 71 presacral region, 82 pelvic sidewalls, 28 perineum, and 5 near the colorectal anastomosis. The five-year LC and OS rates were 88% and 59%, respectively.
Isozaki et al. [Citation15] reported on CIRT for isolated para-aortic lymph node recurrence from rectal cancer in a cohort of 20 patients. The most commonly used dose was 52.8 Gy (RBE) with a median daily dose of 4.4 Gy in 12 fractions. The 3-year OS rate was 57.9%. Of the entire cohort of 34 patients, which included both colon and rectal cancers, there were no acute or late grade 3 or higher toxicities.
Mobaraki et al. [Citation16] published a cost-effectiveness analysis comparing CIRT and multi-modality therapy in patients with LRRC. Eleven patients who underwent combination therapy with 3 D-CRT to a dose of 50–58 Gy, chemotherapy, and hyperthermia were compared to CIRT alone to doses between 70.4 Gy (RBE) to 73.6 Gy (RBE) in 16 fractions over 4 weeks. The 2-year OS rate was 85% for CIRT and 55% for combination treatment arm. The average expenditures for CIRT and multimodality therapy were estimated to be 48,03,946 yuan ($44,235USD) and 46,11,100 yuan ($42,459 USD), respectively, suggesting that CIRT appears to be on par with multi-modal therapy with a superior survival benefit.
Re-irradiation in LRRC
Habermehl et al. [Citation17] reported the first results of re-irradiation in LRRC. Nineteen patients from 2011 to 2013 who had undergone prior pelvic photon radiation to 50 Gy and who were deemed inoperable were included. The dose regimen that was used was 36 Gy (RBE) in 12 fractions over 3 weeks at 3 Gy per fraction. Eight patients received CIRT through a single beam and 11 patients were planned with two beams. The median OS was 9.1 months and the median PFS was 20.6 months. No grade 3 or higher toxicities were observed.
Ongoing clinical trials of CIRT in rectal cancer
PANDORA-01 is a phase I/II study from Heidelberg, Germany aimed at identifying the appropriate dose of CIRT for LRRC with a previous history of pelvic radiation. The phase I study will assess stepwise dose escalation from 12 × 3 GyE to 18 × 3 GyE with toxicity as the primary endpoint. The maximum tolerated dose will be advanced to a phase II study with endpoints as OS, PFS, toxicity and safety [Citation18]. HIMAT1351 is a phase II study from Saga, Japan assessing the role of CIRT in the treatment of patients with local recurrence after primary resection of their rectal cancer without any prior history of radiation [Citation19].
In another study from Chiba, Japan, the authors are assessing the role CIRT for pelvic recurrent rectal cancer in patients with prior pelvic carbon-ion irradiation [Citation20]. Currently, there are no clinical studies assessing the role of CIRT as neoadjuvant therapy in primary rectal cancer.
Discussion
This systematic review provides a cross-sectional view of the outcomes following CIRT for LRRC and an overview of the potential advantages and disadvantages of CIRT in the clinical setting. While the mechanisms of promising outcomes for CIRT for LRRC are not immediately apparent, the preponderance of data suggest that higher biologically effective doses of radiation can be administered safely with hypofractioanted regimens of CIRT.
Carbon ions are heavy particles that induce dense ionization along their track, leading to clustered complex DNA damage, overwhelming the cellular repair mechanisms. Due to a higher LET, there is a greater degree of genomic instability and more effective cell death compared to proton or photon irradiation, which accounts for the higher relative biological effectiveness of 2.5–3 of carbon ions [Citation21–23]. This overwhelming DNA damage of carbon ions is especially beneficial in hypoxic tumors that are traditionally radioresistant because they lack oxygen to fix the DNA damage caused by radiation. Similar to protons, carbon ions also have a relatively low entrance dose, sharp lateral penumbra, and a distinct Bragg peak at tumor depths but suffer from potentially higher exit dose due to a fragmentation tail [Citation24]. The superior physical dose distribution characteristics coupled with a strong radiobiological advantage makes CIRT an attractive therapeutic option for many recurrent cancers following prior radiation therapy.
In LRRC, patients have had prior surgery or radiation therapy or both resulting in disruption of the lymphovascular planes creating a hypoxic radio-resistant and/or chemo-resistant milieu that drives poor treatment outcomes. While this determines chemoradiation responsiveness, surgical management of these tumors is also challenging. An R0 margin is the single most important predictor of survival outcomes; unfortunately, only a sixth of all LRRC patients, typically those with centrally recurrent tumors, are amenable for surgery [Citation25]. In a meta-analysis by Lee et al. [Citation7], patients who had re-irradiation with photons and surgery the three year OS rate was 51.7% and in patients who underwent re-irradiation alone without surgery 3-year OS rates was 23.8%. The acute and late grade 3 complication rates were 11.7% and 25.5%, respectively, in the surgery and photon re-irradiation group vs. the photon re-irradiation alone group. In contrast, the largest CIRT study reported an impressive 3-year OS rate of 73% and 5-year OS rate of 51% with a complication rate of 1.33% and 5.3% for acute and late complications respectively [Citation6]. The seemingly superior outcomes of CIRT may be due to the higher BED (107.5 Gy10) achieved by administering 73.6 Gy(RBE) in 16 fractions compared to the BED (85.7Gy10) achieved with photon re-irradiation, assuming a tumor α/β ratio of 10 [Citation6,Citation7]. Notably, however, despite the high radiation doses used, the reported acute and late complication rates in CIRT were minimal. While these promising results compare favorably with traditional approaches for the treatment of LRRC, additional studies, particularly comparing CIRT to proton or photon radiotherapy are needed before making clinical treatment recommendations. Equally, there is a need to assess carbon ions as part of neoadjuvant treatment in rectal cancer where dose escalation may have a benefit to improved pathological complete response rates, tumor downstaging, and/or consideration of organ-preserving strategies [Citation26].
The main deterrent to the use of CIRT in this recurrent setting is the daunting cost of establishing a carbon ion facility, with an estimated cost of approximately $150 million for a combined particle therapy center [Citation27]. However, as the technology advances and as more centers get established this financial deterrent will be eroded. Additionally, there are only 13 CIRT facilities around the world, with a majority of them in Japan, limiting the accessibility to the majority of patients with LRRC [Citation28]. There are currently more centers under development, with the only center in the United States announced under construction in Jacksonville, Florida. With the opening of this center, we anticipate additional high quality pre-clinical and clinical trials to fully evaluate the role of CIRT in LRRC.
A number of shortcomings of this systematic review warrant discussion. First, most studies besides one are relatively small single-institutional experiences. In many instances, details of treatment field design are lacking. Almost all studies used fixed beam geometries rather than gantry-based CIRT. In some instances, the groups of patients are a mixture of those who received prior radiation and those who did not. These attributes of studies make their conclusions largely hypothesis generating rather than definitive and/or conclusive. Second, the definition of delivered radiation dose is different between German and Japanese studies because of their use of different empirical models to convert physical dose to biologically effective dose. In general, the dose prescription in Germany uses the local effects model whereas in Japan uses the microdosimetric kinetic model or the mixed-beam model. This makes direct comparisons difficult especially when fractional doses are significantly different. Third, the contribution of confounding factors such as patient referral patterns, selection criteria, preexisting comorbidities, choice of concurrent chemotherapy, and differences in reporting guidelines for acute and late toxicities makes it challenging to draw definitive conclusions.
Nevertheless, this first comprehensive systematic review of CIRT for LRRC suggests that favorable outcomes can be achieved in both previously irradiation and un-irradiated tumors and seemingly achieves these outcomes without severe adverse side-effects. While this review identifies trends and common themes across studies, the observations should be considered hypothesis generating rather than conclusive. Taken together, these studies and this review argue strongly for greater collaborative efforts for validation in larger datasets and prospective randomized studies.
Conclusions
CIRT is a promising new technology with mounting evidence of its utility in radioresistant tumors or where re-irradiation is required. The outcomes seen with CIRT in LRRC are promising and merit further exploration in prospective protocols.
Supplemental Material
Download MS Word (13.1 KB)Disclosure statement
The authors have no potential conflict of interest to disclose.
References
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646.
- Martling AL, Holm T, Rutqvist LE, et al. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet (London, England). 2000;356:93–96.
- Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet (London, England). 2009;373:811–820.
- van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet (London, England). 2018;391:2537–2545.
- Shinoto M, Yamada S, Okamoto M, et al. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1404 Rectum. Radiother Oncol. 2019;132:236–240.
- Lee J, Kim CY, Koom WS, et al. Practical effectiveness of re-irradiation with or without surgery for locoregional recurrence of rectal cancer: a meta-analysis and systematic review. Radiother Oncol. 2019;140:10–19.
- Guren MG, Undseth C, Rekstad BL, et al. Reirradiation of locally recurrent rectal cancer: a systematic review. Radiother Oncol. 2014;113:151–157.
- Malouff TD, Peterson JL, Mahajan A, et al. Carbon ion radiotherapy in the treatment of gliomas: a review. J Neurooncol. 2019;145:191–199.
- Mohamad O, Sishc BJ, Saha J, et al. Carbon ion radiotherapy: a review of clinical experiences and preclinical research, with an emphasis on DNA damage/repair. Cancers. 2017;9:66.
- Rackwitz T, Debus J. Clinical applications of proton and carbon ion therapy. Semin Oncol. 2019;46:226–232.
- Particle Therapy Co-operative Group. 2020. Available from: https://www.ptcog.ch/
- Shiba S, Okamoto M, Kiyohara H, et al. Prospective observational study of high-dose carbon-ion radiotherapy for pelvic recurrence of rectal cancer (GUNMA 0801). Front Oncol. 2019;9:702.
- Yamada S, Kamada T, Ebner DK, et al.; Working Group on Locally Recurrent Rectal Cancer. Carbon-ion radiation therapy for pelvic recurrence of rectal cancer. Int J Radiat Oncol Biol Phys. 2016;96:93–101.
- Isozaki Y, Yamada S, Kawashiro S, et al. Carbon-ion radiotherapy for isolated para-aortic lymph node recurrence from colorectal cancer. J Surg Oncol. 2017;116:932–938.
- Mobaraki A, Ohno T, Yamada S, et al. Cost-effectiveness of carbon ion radiation therapy for locally recurrent rectal cancer. Cancer Sci. 2010;101:1834–1839.
- Habermehl D, Wagner M, Ellerbrock M, et al. Reirradiation using carbon ions in patients with locally recurrent rectal cancer at HIT: first results. Ann Surg Oncol. 2015;22:2068–2074.
- Combs SE, Kieser M, Habermehl D, et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: the PANDORA-01 trial. BMC cancer. 2012;12:137.
- A phase II clinical trial of carbon-ion radiotherapy for patients with local recurrence after primarily resected rectal cancer (HIMAT1351). 2014. Available from: https://uploaduminacjp/cgi-open-bin/icdr_e/ctr_viewcgi?recptno=R000015979
- A phase II clinical trial of carbon-ion radiotherapy for pelvic recurrent rectal cancer in patients with prior pelvic carbon-ion irradiation. 2014. Available from: https://uploaduminacjp/cgi-open-bin/ctr_e/ctr_viewcgi?recptno=R000025839
- Chatterjee A, Holley WR. Biochemical mechanisms and clusters of damage for high-LET radiation. Adv Space Res. 1992;12:33–43.
- Sutherland BM, Bennett PV, Schenk H, et al. Clustered DNA damages induced by high and low LET radiation, including heavy ions. Phys Med. 2001;17 :202–204.
- Weyrather WK, Debus J. Particle beams for cancer therapy. Clin Oncol (R Coll Radiol). 2003;15:S23–S28.
- Weber U, Kraft G. Comparison of carbon ions versus protons. Cancer J. 2009;15:325–332.
- Westberg K, Palmer G, Hjern F, et al. Population-based study of factors predicting treatment intention in patients with locally recurrent rectal cancer. Br J Surg. 2017;104:1866–1873.
- Gunther JR, Chadha AS, Shin US, et al. Preoperative radiation dose escalation for rectal cancer using a concomitant boost strategy improves tumor downstaging without increasing toxicity: a matched-pair analysis. Adv Radiat Oncol. 2017;2:455–464.
- Peeters A, Grutters JP, Pijls-Johannesma M, et al. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol. 2010;95:45–53.
- Ebner DK, Kamada T. The emerging role of carbon-ion radiotherapy. Front Oncol. 2016;6:140.