Abstract
Background: Brain metastases (BMs) are an uncommon presentation of metastatic colorectal cancer (mCRC) with reported incidence of about 2–4%. Today, there is an increased awareness towards a metastasis directed treatment approach with either surgical resection, stereotactic radiotherapy (SRT) or both. We examined patient characteristics and survival for patients treated with a localized modality for BM from CRC in a nationwide population-based study.
Methods: A registry-based cohort study of all patients with a resected primary colorectal cancer and localized treatment of BM during 2000–2013. We computed descriptive statistics and analysed overall survival by the Kaplan–Meier method and Cox regression.
Results: A total of 38131 patients had surgery for a primary CRC and 235 patients were recorded with a metastasis directed treatment for BM, comprising resection alone (n = 158), SRT alone (n = 51) and combined resection and SRT (n = 26). Rectal primary tumor (48.9% vs. 36.2%, p < .001) and lung metastasectomy (11.9 vs 2.8%, p < .001) were more frequent in the BM group. The median survival of patients receiving localized treatment for BM was 9.6 months (95% confidence interval (CI) 7.2–10.8). The 1- and 5-year overall survival were 41.7% (95% CI 35–48%) and 11.2% (95% CI 6.9–16.3%). In multivariate analysis, nodal stage was associated with increased mortality with a hazard ratio of 1.63 (95% CI 1.07–2.60, p = .03) for N2 stage with reference to N0.
Conclusion: We report a median overall survival of 9.6 months for patients receiving localized treatment for BM from CRC. Lung metastases and rectal primary tumor are more common in the population treated for BM.
Introduction
Colorectal cancer (CRC) is one of the major causes of cancer-related mortality and morbidity with a frequent pattern of metastasis involving liver, lung and/or the peritoneal cavity [Citation1,Citation2]. Brain metastases (BMs) from CRC are a relatively rare manifestation with reported incidence ranging from 2 to 4%, in contrast to patients with lung cancer, breast cancer or melanoma, where BMs are frequently encountered [Citation3,Citation4]. A large proportion of patients with BM from CRC receive symptom-directed palliative treatment including whole brain radiation, steroids and supportive care with an expected median survival of a few months. However, several retrospective studies have demonstrated a subset of patients subjected to metastasis directed treatment, might be long-term survivors [Citation5–7].
Surgical resection of BM in highly selected patients with metastatic cancer has been performed for decades and is supported by the first randomized study in 1990 [Citation8] where patients with a solitary BM, across all types of solid tumours, were randomized to either radiotherapy or resection, with a significant improved survival for the latter group. Stereotactic Radiotherapy (SRT) is a high precision external radiotherapy with the delivery of a few fractions with a high dose of energy [Citation9]. Similar to surgical resection, the addition of SRT to whole brain radiation has shown a superior outcome for selected patients with up to three BMs [Citation10].
Following multidisciplinary assessment, resection and/or SRT are considered a standard of care for appropriately selected patients with BM across various solid tumours. We examined patient characteristics, survival and prognostic factors in a population-based cohort study for patients receiving localized treatment (resection and/or SRT) for BM from CRC.
Material and methods
Data sources
From the Danish national administrative and medical registries, we retrieved data applying the unique civil registration number assigned to all Danes at birth or upon immigration [Citation11]. Across all registries, this number allows for accurate identification at individual level. Since 1977, the Danish National Patient Registry (DNPR) holds all information on diagnosis, procedures and hospitalization, and since 1995 including emergency room visits and outpatient contacts [Citation12].
In Denmark, all cases of cancer are compulsory reported to the Danish Cancer Registry (DCR) since 1943 using the International Classification of Disease (ICD) [Citation13].
Since 1997, all pathological examinations and diagnoses are recorded in the Danish Pathology Registry (DPR) using the Danish Systematized Nomenclature of Medicine (SNOMED) codes [Citation14]. The survival status of all Danish citizens is retrieved from the Danish Civil Registration System, with accurate recording of vital status since April 1968 [Citation11].
Design and population
We retrieved information on all patients resected for a primary colorectal adenocarcinoma from 1 January 2000 to 31 December 2013 recorded in the DNPR covering all stages I–IV.
From this cohort, we identified all patients whom had undergone surgical resection of BM or had received cranial SRT. We used the DCR to confirm the diagnosis of CRC and to exclude all patients with other primary cancer diagnoses except non-melanoma skin cancer at the date of CRC. From DPR, we confirmed the adenocarcinoma histology of all surgical specimens.
Variables
Comobidity was quantified by the Charlson Comorbidity Index (CCI) [Citation15]. The CCI is a validated index, where patients are assigned between 1 and 6 points for various conditions and disorders. For each patient, a sum of score was calculated based on the presence of specific comorbid conditions diagnosed at the time of CRC surgery, excluding malignancy from the index. This allows managing CCI as a categorical variable: no comorbidity (CCI = score 0), minor comorbidity (CCI = score 1–2) and severe comorbidity (CCI = score 3+).
Follow-up and outcomes
We calculated overall survival from the date of BM treatment until death of any cause, emigration, or censoring at end of follow-up (31 December 2014). For patients receiving both resection and SRT at any point during the study period, survival was calculated from the last treatment modality applied.
Statistical analysis
For descriptive statistics, we report continuous variables by median values, range and interquartile range (IQR). Categorical variables are shown as numbers/percentages and compared by cross tabulation and chi2 test.
For analysis of survival, we used the Kaplan–Meier method. For comparison of survival between groups, we applied Cox proportional hazards regression to calculate hazard ratios (HR) as a measure of the mortality rate ratio. We included the clinical relevant parameters age, gender, site of primary, TN stage of primary, year of BM treatment, CCI and treatment modality separately and in a multivariable model. The log-log plot was inspected confirming the proportionality assumption behind the Cox model.
All calculations were done by STATA version 14.1 (StataCorp, College Station, TX).
Ethics
The study was approved by the Danish Data Protection Agency (record no. 2014-41-3344]). In Denmark registry studies with no patient contact do not require ethical approval and all data were de-identified and handled anonymously.
Results
Patient characteristics
During 2000–2013, a total of 38,131 patients were resected for a primary CRC and from this group 235 patients underwent a localized treatment for BM, comprising resection alone (n = 158), SBRT alone (n = 51) and combined resection and SRT (n = 26). For the BM treatment cohort, males constituted 49.8% and the median age was 64.8 years (IQR 57.3–71.1 years). Patient characteristics are display in . Further descriptive statistics for the cohort of patients resected for a CRC has been published previously [Citation16].
Table 1. Characteristics of patients resected for a colorectal cancer who have received a localized treatment for BMs during 2000–2013 (central column).
Comparison with patients resected for primary tumors
Compared to all patients resected for a primary CRC, patients receiving a localized BM treatment were younger and with female predominance. The ratio of rectal/colonic cancer was 48.9%/51.1% for the BM treatment group (n = 235) compared to 36.3%/63.8% for the population of patients with a resected primary tumour (n = 38,131) (p < .001). Similarly, 11.9% of patients in the BM treatment group were resected for lung metastases while 2.8% of all resected CRC patients underwent lung metastasectomy (p < .001). The comorbidity burden was well balanced between the two groups and patients in the BM group tended to have a higher nodal (N) stage of the primary compared to patients with a resected primary.
Survival and prognostic factors
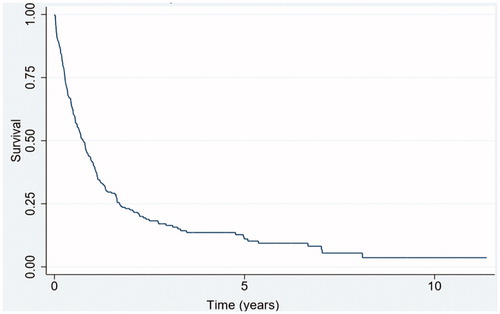
A total of 212 deaths were recorded with a median follow-up of 3.3 years (95% CI 2.8–3.6). The median survival was 9.6 months (95% CI 7.2–10.8) for patients treated with a localized modality for BM (n = 235). The 1- and 5-year overall survival were 41.7% (95% CI 35.4–47.9%) and 11.2% (95% CI 6.9–16.3%), respectively ().
Figure 1. Overall survival from time of localized treatment of BMs during 2000–2013 for Danish patients resected for a colorectal adenocarcinoma (n = 235).
In both uni- and multivariate analysis (), N2 stage of the primary tumour was associated with increased mortality with a HR of 1.74 (95% CI 1.16–2.65) and 1.63 (1.07–2.60, p = .03), respectively, with reference to N0. The T stage was not related to survival.
Table 2. Uni- and multivariate analysis showing Hazard Ratios (HR) with 95% confidence intervals (CIs) for mortality for 235 Danish patients receiving localized invasive treatment for BMs from colorectal cancer.
Surprisingly, patients with minor comorbidity (CCI 1–2) had an adjusted HR for mortality of 0.64 (95% CI 0.45–0.92) compared to patients with no comorbidity (CCI 0). Severe comorbidity (CCI 3), gender, age and site of primary were not associated with prognostic impact.
Patients only treated with SRT had an increased mortality with a HR of 2.01 (95% CI 1.44–2.88) and 2.12 (95% CI 1.48–3.03) in uni- and multivariate analysis compared to patients treated with BM resection alone. Patients receiving combined modality treatment had a non-significant tendency for improved survival compared to patients only receiving resection with a HR of 0.69 (95% CI 0.43–1.11).
Discussion
The overall survival of patients with metastatic CRC has gradually improved over the past decades with improved surgery, radiotherapy and use of cytotoxic agents [Citation17,Citation18]. BMs from CRC are a poorly understood phenomenon and often reported to be an end stage metastatic condition. In this population-based study, we found a median overall survival of 9.6 months for patients who have undergone a localized BM treatment (e.g., resection or SRT). This is in concordance with what has previously been reported in case series, limited by a small sample size [Citation5–7].
Interestingly, the pattern of the survival curve from our study () shares characteristics with the above-mentioned case series, e.g., a rapid decline with a median overall survival less than 1 year, followed by a plateau phase in the tail of the curve, suggesting a subgroup of patients are long term survivors. However, with the relatively large population in this registry-based study, we estimated a 5-year survival of 11.2% for resected CRC patients with resected or SRT treated BM.
It should be stressed, that we only have data available for patients with BM who have undergone a metastasis directed treatment modality. The survival for the majority of colorectal cancer patients with BM, undergoing a symptomatic palliative course cannot be accounted for in this study.
We found the nodal stage of the primary tumour to be an independent prognostic marker, as patients with N2 stage had a HR for mortality of 1.63 (1.07–2.60) in multivariate model with reference to N0. The negative prognostic impact of increasing nodal stage is well established for patients undergoing metastasis directed treatment for liver and/or lung metastases [Citation16,Citation19]. Thus, this finding is not unique for the group of BM treatment, instead the nodal stage can be considered a surrogate marker for a more aggressive tumour biology.
We noted a surprising and controversial association between co-morbidity and survival, where patients with minor co-morbidity had an improved survival. This apparent paradoxical finding should be interpreted with caution, due to the small number of patients with CCI 1–2 (n = 62) and CCI 3+ (n = 36). Due to the short median survival (9.6 months) for this population, it is possible the impact of a co-morbidity burden is minor than for other colorectal cancer populations with longer survival [Citation16].
Previous studies have demonstrated an association between lung metastases and rectal site of the primary tumour with the occurrence of BM [Citation20]. Compared to a reference population of patients with a resected primary CRC, we confirm a more frequent occurrence of rectal cancer, compared to colonic cancer, for patients treated for BM. Similarly, the use of lung metastasectomy was more common in this group, also suggesting a biological connection between BM and lung metastases.
We have identified one other registry-based study addressing BM from CRC. The South Australian metastatic colorectal cancer (SAmCRC) registry [Citation21] contains data for 4100 patients with metastatic CRC. From here, 59 patients (1.4%) were recorded with BM and patients undergoing a craniotomy had a median survival of 8.5 months. Similar to our data, they found female gender, younger age and rectal primary to be associated with BM. The SAmCRC registry also holds molecular characteristics and shows more frequently KRAS mutation for patients with BM (55% vs 42%).
As a registry-based study, using the Danish nationwide medical registries, there are limitations to this study. We lack details on individual treatment courses and imaging. Similarly, potential confounding factors such as lifestyle factors and socio-economic status cannot be adjusted for as well as medical data not recorded in DNPR e.g., the use of whole-brain radiation and steroid treatment. However, the validity of data from the Danish Cancer Registry has been shown to be consistently high, due to a systematic and compulsory registration of all cancer diagnoses in the Danish healthcare administration [Citation22]. With a nationwide universal health care system in Denmark, treatment is not restricted to selected patients with a private health insurance. Furthermore, with the Danish Pathology Registry, we are able to exclude patients with different pathology, e.g., primary brain tumours. Finally, examining patients with BM, data regarding quality of life and performance status deterioration would be beneficial but are not retrievable in the medical registries.
A treatment approach with a more aggressive strategy for BM is supported by a recent review [Citation23] and endorsed by the updated clinical guidelines from the Danish National board of Health [Citation24]. Here, it is recommended that all patients with up to 4 metastases always are evaluated by a multidisciplinary tumour board with neurosurgical and radiation expertise.
In conclusion, we report an association between rectal site of primary tumour and lung metastasectomy with localized treatment for BMs from colorectal cancer. We found a median overall survival of 9.6 months and an estimated 5-year survival as high as 11.2%. Increasing nodal stage of the primary tumour is associated with increased mortality.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Robinson JR, Newcomb PA, Hardikar S, et al. Stage IV colorectal cancer primary site and patterns of distant metastasis. Cancer Epidemiol. 2017;48:92–95.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136(5):E359–E386.
- Christensen TD, Spindler KL, Palshof J, et al. Systematic review: brain metastases from colorectalcancer – incidence and patient characteristics. BMC Cancer. 2016;16:260.
- Lemke J, Scheele J, Kapapa T, et al. Brain metastases in gastrointestinal cancers: is there a role for surgery?. IJMS. 2014;15(9):16816–16830.
- Gu X-D, Cai Y-T, Zhou Y-M, et al. Prognostic factors and multidisciplinary treatment modalities for brain metastases from colorectal cancer: analysis of 93 patients. BMC Cancer. 2015;15:902.
- Michl M, Thurmaier J, Schubert-Fritschle G, et al. Brain metastasis in colorectal cancer patients: survival and analysis of prognostic factors. Clin Colorectal Cancer. 2015;14(4):281–290.
- Mege D, Ouaissi M, Fuks D, et al. Patients with brain metastases from colorectal cancer are not condemned. Anticancer Res. 2013;33:5645–5648.
- Patchell R, Tibbs P, Walsh J, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500.
- Leth T, v Oettingen G, Lassen Y, et al. Survival and prognostic factors in patients treated with stereotactic radiotherapy for brain metastases. Acta Oncol. 2015;54(1):107–114.
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672.
- Pedersen CB, Gotzsche H, Moller J, et al. The Danish Civil Registration System. S cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–33.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–45.
- Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39(7 Suppl):72–74.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Boysen AK, Spindler KL, Høyer M, et al. Metastasis directed therapy for liver and lung metastases from colorectal cancer-A population-based study. Int J Cancer. 2018;143(12):3218–3226.
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683.
- Iversen LH, Green A, Ingeholm P, et al. Improved survival of colorectal cancer in Denmark during 2001-2012 – the efforts of several national initiatives. Acta Oncol. 2016;55(Suppl 2):10–23.
- Fong Y, Fortner J, Sun R, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Ann Surg. 1999;230(3):309–321.
- Christensen TD, Palshof JA, Larsen FO, et al. Risk factors for brain metastases in patients with metastatic colorectal cancer. Acta Oncol. 2017;56(5):639–645.
- Tapia G, Price T, Karapetis C, et al. Brain metastases in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC) registry. Cancer Biol Med. 2017;14:371–376.
- Thygesen SK, Christiansen CF, Christiansen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83.
- Mege D, Sans A, Ouaissi M, et al. Brain metastases from colorectal cancer: characteristics and management. ANZ J Surg. 2018;88(3):140–145.
- Danish National Board of Health. 2018. Treatment of brain metastases. Available from: https://www.sst.dk/da/udgivelser/2018/nkr-behandling-af-hjernemetastaser
