Abstract
Background: The UICC TNM 7th edition introduced stage groups for anal cancer which in 2019 has not yet come into general use. The new TNM 8th edition from 2016 defines 7 sub-stages. Background data for these changes are lacking. We aimed to investigate whether the new classification for anal cancer reliably predict the prognosis in the different stages.
Patients and methods: The Nordic Anal Cancer Group (NOAC) conducted a large retrospective study of all anal cancers in Norway, Sweden and most of Denmark in 2000–2007. From the Nordic cohort 1151 anal cancer patients with follow-up data were classified by the TNM 4th edition which has identical T, N and M definitions as the TNM 7th edition, and therefore also can be classified by the TNM 7th stage groups. We used the Nordic cohort to translate the T, N and M stages into the TNM 8th stages and sub-stages. Overall survival for each stage was assessed.
Results: Although the summary stage groups for TNM 8th edition discriminates patients with different prognosis reasonably well, the analyses of the seven sub-stages show overlapping overall survival: HR for stage IIA 1.30 (95%CI 0.80–2.12) is not significantly different from stage I (p = .30) and HR for stage IIB 2.35 (95%CI 1.40–3.95) and IIIA 2.48 (95%CI 1.43–4.31) are also similar as were HRs for stage IIIB 3.41 (95%CI 1.99–5.85) and IIIC 3.22 (95%CI 1.99–5.20). Similar overlapping was shown for local recurrence and distant spread.
Conclusion: The results for the sub-stages calls for a revision of the staging system. We propose a modification of the TNM 8th edition for staging of anal cancer into four stages based on the T, N and M definitions of the TNM 8th classification.
Introduction
Anal cancer is a rare tumour entity with rising incidence [Citation1]. Globally the disease is diagnosed in 27,000 new cases each year [Citation2]. The clinical outcome is very good for early stages, but for locally advanced stages the recurrence rates and survival give room for improvements.
Tumour size and nodal status have generally been used separately as prognostic factors for anal cancer, but also combined as tumour and node status (TN categories) for patients without distant metastases at presentation [Citation3,Citation4]. The composite TNM stages have not been generally used for anal cancer, in contrast to e.g., colorectal cancer where the TNM stage is the accepted standard classification in clinical and scientific reports.
The Union for International Cancer Control (UICC) TNM Classification of Malignant Tumours 8th edition (TNM8) published in 2016, introduced several changes in the lymph node classification for anal cancer [Citation5]. Anal margin cancer within 5 cm from anal verge is now classified as anal cancer and not skin cancer. The T classification was unchanged. The external iliac lymph nodes are considered as a site of regional disease (N), previously they were not specified and could be classified as a site of distal metastasis (M). Lymph node status was previously recorded as N0 and N1–N3 in the TNM 7th edition (TNM7) [Citation6]. It is now divided into two main cathegories: N0 and N1 as used by Ajani et al., however with a new subclassification into 1a, 1b, and 1c [Citation5–7]. Note that N1a may include multiple malignant lymph nodes in several regional locations as long as the external iliac nodes are not involved. Furthermore, the TNM8 introduces additional stage sub-categories relative to TNM7: Stage II is divided into IIA and IIB depending on T stage, while stage III is grouped in three, IIIA, IIIB, and IIIC depending on T and N stage (). Subtle changes have also been made in which stage the different combinations of T and N status belong [Citation5,Citation6]. In general, the prognostic importance of nodal disease involvement has been downplayed, while T3 or T4 status is considered more severe. To our knowledge, the changes implemented in the TNM8 classification and staging have not been validated by any clinical prognostic data except the division of stage II [Citation8].
Table 1. Changes from the TNM 7th edition to the TNM 8th edition for classification of anal cancer.
We here retrospectively apply the TNM 8th edition staging system for classifying a large cohort of 1151 patients from the Nordic countries [Citation9] previously classified by the TNM 4th edition (TNM4). TNM4 for anal cancer is identical to TNM7 regarding what constitutes T, N and M, but lacks the summary stages included in TNM7 [Citation10].
Methods and patients
The Nordic study
The Nordic Anal Cancer Group (NOAC) conducted a large retrospective study of all anal cancers (including squamous cell cancer, cloacogenic and basaloid cancer; excluding adenocarcinomas) admitted to oncology departments in Sweden and Norway and Herlev University Hospital, Vejle University Hospital and Odense University Hospital in Denmark from July 2000 to June 2007. The standard examination included proctoscopy with biopsy, computer tomography (CT) of chest and abdomen, and magnetic resonance imaging (MRI) of the pelvic area was gradually introduced during the study period. CT-(18)F-fluorodeoxyglucose positron emission tomography (FDG-PET), CT-PET was not used. The majority of the patients were treated according to one of seven different NOAC protocols (NOAC 1–7), the treatments given are summarised in [Citation9]. Radiation dose to primary tumour and involved nodes varied between 54 and 60 Gy in combination with chemotherapy according to the stage and protocols, others were treated with radiation (54–64 Gy) or surgery alone in early stages. Elective lymph node regions received a radiation dose of 42–46 Gy, except in some centres for well differentiated T1N0 tumours, where elective inguinal lymph node irradiation was omitted. Different chemotherapy schedules were used, either one or two courses of 5-fluorouracil and mitomycin C concurrent with the radiation, or three cycles of cisplatin and 5-fluorouracil given prior to the radiation or two cycles prior and the third cycle concurrent with radiation.
Table 2. Patient characteristics in the Nordic NOAC database of anal cancer.
From the database with 1266 patients we excluded 115 patients due to early stages (T0 n = 1, Tis n = 6), lack of proper metastasis classification (MX n = 61), lack of proper local stage assessment (TX or NX n = 42) and lack of follow-up data (n = 5), leaving 1151 patients with details on the primary TNM stage, treatment and follow-up. The patients had a median follow-up period of 7.2 years (range 6.5–7.9).
The patients were classified according to TNM 4th edition [Citation10], which is identical to the TNM 7th edition [Citation6]. Note that we treated external iliac lymph node metastases that could be included in standard radiotherapy volumes as locoregional disease and not as M1. These patients were therefore restaged as N1M0 in the TNM8 classification, and thus can be retrospectively correctly classified according to the new stages of TNM8. Also, skin squamous cell cancers within 5 cm from the anal verge were treated as anal cancers, in accordance with the current TNM8. A retrospective assessment of the TNM 8th edition stage classification was therefore considered appropriate using the Nordic NOAC cohort.
Statistics
The primary endpoint was overall survival. Survival was defined as the time from diagnosis to death of any cause or censored at last follow-up. Survival was estimated by the Kaplan Meier method, and differences tested by the log-rank method using the IBM SPSS 25 package (IBM Corp., Armonk, NY, USA). A two-tailed p-value below .05 was considered statistically significant. Hazard ratios with 95% confidence intervals (95%CI) were calculated by Cox regression proportional hazards model using SPSS 25.
The NOAC study received ethics approval in all Nordic countries including patients.
Results
Results of the Nordic cohort
For clinical assessment of the prognosis the T category is central. In the Nordic cohort there were small differences by T categories in rate of locoregional recurrence: T1 15%, T2 14%, T3 16%, and T4 15%. The risk of combined locoregional failure and distant metastases was for the T stages 1%, 3%, 5% and 5%, respectively. Slightly larger differences were observed for the risk of distant metastases: T1 4%, T2 4%, T3 7%, and T4 11%.
The main assessment of nodal status for anal cancer in the TNM8 classification is whether there is clinical or imaging signs of nodal metastases at all or not. We do not have access to a prospective classification according to the TNM8 classification of lymph node involvement. As a proxy we therefore analysed the prognostic prediction of the nodal classification of the TNM7 (identical to TNM4). The local recurrence rates did not change much by N-stage (range 13 – 16% locoregional only, and 15 – 23% with distant metastases), while distant metastases without or with local recurrences varied more: N0 4%, N1 7%, N2 10%, and N3 15%. The total recurrence rate in stage N0 was 19%, N1 23%, N2 33%, and N3 35%, respectively.
Results after reclassification
The distribution of the patients according to the TNM7 and TNM8 staging is shown in . Stage I and stage IV are unchanged in the new classification. External iliac lymph node metastases are regarded as M1 according to TNM7 (and TNM4) classification, but in our NOAC series they were recorded (and treated) as locoregional disease as in TNM8. For patients classified as stage II in TNM7, 72% are classified in TNM8 as stage IIA while 28% are allocated to stage IIB. In TNM7 stage IIIA, 18% remained in TNM8 as stage IIIA, while 50% were recorded as stage IIIB and 32% stage IIIC. None of the patients classified as TNM7 stage IIIB remained in this category in TNM8: 26% were classified as stage IIIA while the remaining 74% were classified as stage IIIC.
Table 3. Correlation between anal cancer stage according to the previous TNM 7th edition and the new TNM 8th edition.
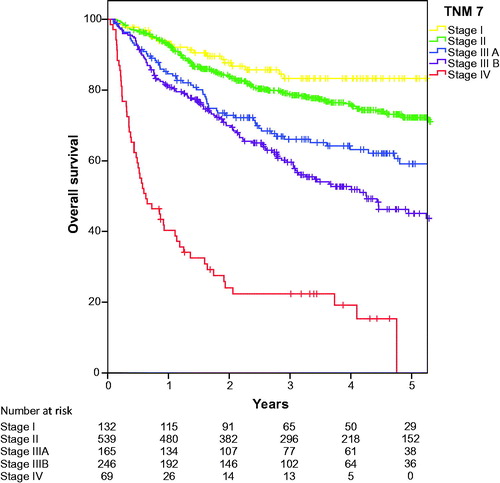
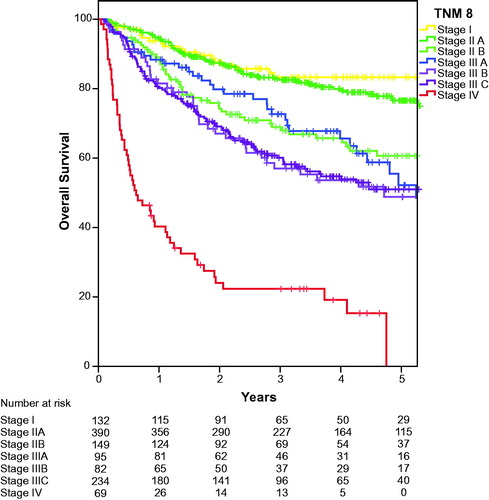
Overall survival according to the crude TNM7 stage classification is shown in . The overall survival for the TNM7 substages is presented in . Hazard ratio (HR) for Stage II was 1.56 (95%CI 0.98–2.50 (p = .62), not significantly different from Stage I, while the other stages were significantly inferior to stage I with HR 2.57 (95%CI 1.55–4.26), 3.45 (95%CI 2.15–5.55 and 12.34 (95%CI 7.38–20.64), for stage IIIA, stage IIIB and stage IV (p < .000 for all), respectively. Overall survival according to crude TNM8 stage classification is identical to survival presented in as we already treated external iliac lymph nodes as N stage and not M1. At first glance, the new classification therefore seems to predict prognosis well. However, when evaluating the survival of the substages in TNM8, as shown in , it becomes clear that both Stage I and Stage IIA as well as Stage IIB and Stage IIIA partly overlap, and that stage IIIB and stage IIIC also have similar estimated survival. HR for stage IIA 1.30 (95%CI 0. 80–2.12) is not significantly different from stage I (p = .29) and HR for stage IIB and IIIA are also similar as were HRs for stage IIIB and IIIC, see . We also analysed the local recurrences and distant spread according to the TNM8 sub-stages and found similar overlapping curves (data not shown).
Figure 1. Overall survival for anal cancer in the NOAC study, classified by TNM7 stages. As we treated positive external iliac lymph nodes as regional spread, we assume that TNM8 stages are practically identical.
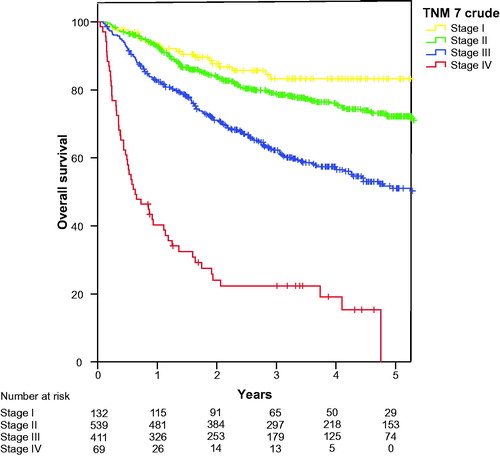
Table 4. Cox regression analysis of the influence of TNM8 stage on hazard ratios for overall survival of anal cancer in the NOAC study.
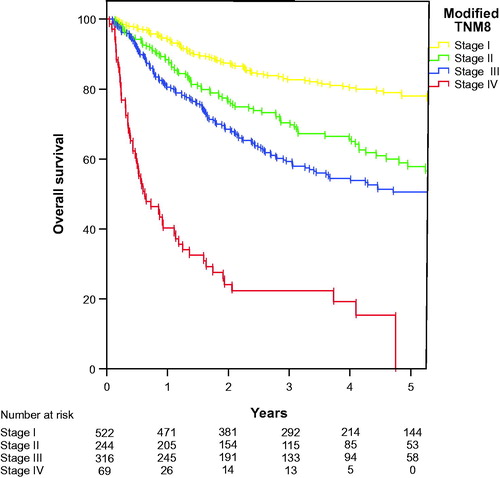
Based on the data presented we propose a modified staging based on the TNM8 subclassification where stage I and stage IIA, stage IIB and stage IIIA, and stage IIIB and stage IIIC, are merged into three categories for anal cancers without metastases and a fourth stage for patients presenting with metastases, see .
Discussion
The TNM classification system serves several purposes, firstly to define tumour extent at the time of diagnosis, serving as the main basis for selection of appropriate therapy, secondly as a prognostic tool to assess the predicted outcome, and thirdly to compare different patient series [Citation11]. To our knowledge this is the first attempt to assess of the ability of the TNM 8th edition sub-classification to separate anal cancer patients into prognostic groups. Being a rare disease in most institutions, a classification system with seven categories may be difficult to handle. Our analysis indicates that the prognostic difference between the separate substages seems better in the previous TNM 7th edition than in the recent TNM 8th edition where survival in several of the substages are overlapping ( and ). The proposed modified four stage system () may be more suitable for clinical use, but it needs of course validation in an independent prospective cohort.
The TNM summary or ‘stages’ introduced in the TNM7 edition have, as far as we understand from reading articles about treatment outcomes in anal cancer, and informal contacts, not been generally used in clinical practice for anal cancer. Instead separate analyses of T and N categories are still used [Citation4,Citation12]. Our analyses show that the prognostic prediction by T and N stage alone is limited when treatment is adjusted to the different T and N stages.
Recently a lymph node stage migration for anal cancer has been documented as a result of the use of MRI for detection of the primary tumour and regional lymph nodes and CT-FDG-PET for visualisation of malignant deposits [Citation13]. The role of CT is today primarily to detect distant metastases and for planning of radiation therapy. Note that N0 status had similar recurrence rates as N1 in the NOAC cohort. This may be related to some degree of underdiagnosis of nodal metastases as neither MRI nor FDG-PET was in general use when recruitment started for the NOAC cohort. PET may detect more lymph nodes, but we need larger series to assess its impact on staging and prognosis for anal cancer patients [Citation14]. The NOAC protocols also included more intensive therapy in higher stages, thus prescribing a risk adapted strategy which may mask real differences. Thus the different radiation doses and chemotherapy regimens may also to some degree have had an impact on the results presented.
The division of TNM8 substage II in A and B has been validated in a large American database study including 8428 anal squamous cell cancer patients, which reported 15% and 19% difference in 5 year overall survival for the National Cancer Database and Surveillance, Epidemiology, and End Results databases, respectively [Citation8]. The stage IIA and B patients were not related to stage I and Stage III patients in this study. Our results confirm their data as we report 16% difference in 5 year overall survival from 60.6% (95%CI 59.7–61.5%) to 76.6% (95% CI 76.1–77.1%), respectively for stage IIA and stage IIB.
Based on the presented data TNM8th stage I and IIA have an excellent prognosis. Due to the overall toxicity and quality of life issues after combined radiation and chemotherapy for anal cancer [Citation15–17] the selection of stage I (T1N0) as candidates for reduction of radiation dose can be endorsed [Citation18]. It remains to be shown whether stage IIA can be treated similarly.
Anal cancer patients presenting with TNM8 stage IIIB and C have approximately 40% risk of recurrence and death after treatment according to current guidelines (Stage III in ). These patients are clearly candidates to test new treatments to improve outcomes.
The strength of the current study is the large sample size (1151 patients) and that it includes all patients referred for oncological therapy on national or regional levels (including > 90% of all patients with anal cancer in Sweden and Norway). A limitation is that all patients were not examined with MRI and that the external iliac lymph nodes were not specifically registered and may occasionally have been regarded as metastatic disease in some centres according to TNM4 (and TNM7).
Presence of human papilloma virus type 16, HPV16, and expression of cyclin-dependent kinase inhibitor 2A (CDKN2A or p16INK4a), in tumour tissues are documented prognostic factors in anal cancer [Citation19–22]. Patients with p16 negative primary tumours generally have poor prognosis. The level of infiltration of lymphocytes in tumour tissues was also shown as a prognostic marker [Citation23]. These molecular classifiers have been proposed to select patients suitable for immunotherapy with immune checkpoint inhibitors in advanced anal cancer [Citation20,Citation24]. The HPV16 and p16 markers predict poor survival [Citation22], but seem in some series primarily to predict for local recurrence. Introduction of molecular factors may in the future further define prognostic groups.
Conclusion
The TNM 8th edition classification has kept earlier T staging and the distinction between N0 and N1 with sub-classification 0, 1a, 1b and 1c. The new classification presents a more detailed stage classification including seven categories which partly overlap with respect to prognostic impact when tested in a large clinical cohort. We therefore introduce a simpler modified four stage system based on the T and N categories of the TNM 8th edition combined. This may serve as a practical tool for clinical assessment of prognosis for newly diagnosed anal cancer patients, but these results need verification in a prospective cohort.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Guren MG, Aagnes B, Nygard M, et al. Rising incidence and improved survival of anal squamous cell carcinoma in Norway, 1987–2016. Clin Colorectal Cancer. 2019;18(1):e96–e103.
- Islami F, Ferlay J, Lortet-Tieulent J, et al. International trends in anal cancer incidence rates. Int J Epidemiol. 2017;46(3):924–938.
- Bentzen AG, Guren MG, Wanderas EH, et al. Chemoradiotherapy of anal carcinoma: survival and recurrence in an unselected national cohort. Int J Radiat Oncol Biol Phys. 2012;83(2):e173–e180.
- Gunderson LL, Moughan J, Ajani JA, et al. Anal carcinoma: impact of TN category of disease on survival, disease relapse, and colostomy failure in US Gastrointestinal Intergroup RTOG 98-11 phase 3 trial. Int J Radiat Oncol Biol Phys. 2013;87(4):638–645.
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. Hoboken (NJ): Wiley-Blackwell; 2016.
- Sobin L, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 7th ed. Chichester (UK): Wiley-Blackwell; 2010.
- Ajani JA, Winter KA, Gunderson LL, et al. Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: the intergroup trial (RTOG 98-11). Cancer. 2010;116(17):4007–4013.
- Goffredo P, Garancini M, Robinson TJ, et al. A national-level validation of the New American Joint Committee on Cancer 8th Edition Subclassification of Stage IIA and B Anal Squamous Cell Cancer. Ann Surg Oncol. 2018;25(6):1654–1660.
- Leon O, Guren M, Hagberg O, et al. Anal carcinoma – survival and recurrence in a large cohort of patients treated according to Nordic guidelines. Radiother Oncol. 2014;113(3):352–358.
- Hermanek P, Sobin LH. TNM classificationn of malignant tumours: UICC. Berlin: Springer Verlag; 1987.
- O’Sullivan B, Brierley J, Byrd D, et al. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. 2017;18(7):849–851.
- Martin D, Rodel C, Fokas E. Chemoradiotherapy for anal cancer: are we as good as we think? Strahlenther Onkol. 2019;195(5):369–373.
- Sekhar H, Zwahlen M, Trelle S, et al. Nodal stage migration and prognosis in anal cancer: a systematic review, meta-regression, and simulation study. Lancet Oncol. 2017;18(10):1348–1359.
- Rusten E, Rekstad BL, Undseth C, et al. Anal cancer chemoradiotherapy outcome prediction using 18F-fluorodeoxyglucose positron emission tomography and clinicopathological factors. Br J Radiol. 2019;92(1097):20181006.
- Bentzen AG, Balteskard L, Wanderas EH, et al. Impaired health-related quality of life after chemoradiotherapy for anal cancer: late effects in a national cohort of 128 survivors. Acta Oncol. 2013;52(4):736–744.
- Bentzen AG, Guren MG, Vonen B, et al. Faecal incontinence after chemoradiotherapy in anal cancer survivors: long-term results of a national cohort. Radiother Oncol. 2013;108(1):55–60.
- Jones CM, Adams R, Downing A, et al. Toxicity, tolerability, and compliance of concurrent capecitabine or 5-fluorouracil in radical management of anal cancer with single-dose mitomycin-C and intensity modulated radiation therapy: evaluation of a national cohort. Int J Radiat Oncol Biol Phys. 2018;101(5):1202–1211.
- Renehan AG, Muirhead R, Berkman L, et al.; Ptm group. Early stage anal margin cancer: towards evidence-based management. Colorectal Dis. 2019;21(4):387–391.
- Balermpas P, Martin D, Wieland U, et al. Human papilloma virus load and PD-1/PD-L1, CD8+ and FOXP3 in anal cancer patients treated with chemoradiotherapy: rationale for immunotherapy. Oncoimmunology. 2017;6(3):e1288331.
- Jones CM, Goh V, Sebag-Montefiore D, et al. Biomarkers in anal cancer: from biological understanding to stratified treatment. Br J Cancer. 2017;116(2):156–162.
- Rodel F, Wieland U, Fraunholz I, et al. Human papillomavirus DNA load and p16INK4a expression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Int J Cancer. 2015;136(2):278–288.
- Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, et al. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol. 2014;32(17):1812–1817.
- Gilbert DC, Serup-Hansen E, Linnemann D, et al. Tumour-infiltrating lymphocyte scores effectively stratify outcomes over and above p16 post chemo-radiotherapy in anal cancer. Br J Cancer. 2016;114(2):134–137.
- Martin D, Rodel F, Balermpas P, et al. The immune microenvironment and HPV in anal cancer: Rationale to complement chemoradiation with immunotherapy. Biochim Biophys Acta Rev Cancer. 2017;1868(1):221–230.