Introduction
Adjuvant chemotherapy for stage III colorectal cancer is recommended by international and Danish guidelines, and reduces recurrence rate and mortality compared with surgery alone, even in selected older patients [Citation1,Citation2]. Chemotherapy has in many studies shown to have the same overall toxicity in older patients [Citation3,Citation4] and even with adjustments in regimens and doses, older patients still have a survival benefit of chemotherapy [Citation5]. However, several previous studies have shown that elderly patients more often undergo only palliative surgery, have less extensive surgery, and are less often referred for adjuvant and neoadjuvant oncological treatment [Citation6,Citation7]. Although the population gets older, the residual life expectancy and the proportion of good years of the residual life expectancy are increasing, as well as the proportion of older people with good mobility, activities of daily living, cognitive function and good health status [Citation8,Citation9]. Further, the surgical treatment of colorectal cancer has improved markedly in the recent decade, with the introduction of centralized treatment, multidisciplinary team (MDT) conferences, enhanced recovery after surgery (ERAS) and minimally invasive surgery. Thus, the overall survival of patients with colorectal cancer in Denmark has improved markedly in recent years [Citation10]. The long-term survival of octogenarians who underwent surgical treatment for colorectal cancer without complications was 5.5 years [Citation11]. However, this was between 2008 and 2011, where only 30% had minimally invasive surgery. Updated studies are needed to explore the referral to oncological adjuvant therapy under the current treatment paradigm and the increase in the proportion of healthy elderly patients.
The aim of this nation-wide population-based prospective cohort study was to investigate the association between age and referral to an oncologist for postoperative adjuvant chemotherapy in patients with resected stage III colorectal cancer in Denmark from 2014 to 2016.
Material and methods
Design and sources of data
The study was conducted as a nationwide cohort study with prospectively collected data from a National Clinical Database maintained by the Danish Colorectal Cancer Group [Citation12]. All patients diagnosed with first time colorectal adenocarcinoma in Denmark are registered in the database and contain both demographic, surgical, pathology and treatment characteristics. The external validity of the database is high due to the high completeness of incident cases (99%) [Citation12]. The study period was the 1 January 2014 to 31 December 2016.
Ethical approval
This study was conducted as a database study and according to Danish law, permission from The National Committee on Health Research Ethics is not necessary. The study was approved by the Danish Data Protection (REG-129-2017).
Study population
The study population included all patients above 18 years of age, who underwent potentially curative, elective surgery for adenocarcinoma in colon or rectum and with UICC stage III. Exclusion criteria were primary endoscopic or other surgical techniques that did not require skin incision, palliative surgery only, emergency surgery, neoadjuvant oncological treatment, unknown stage of cancer, and death within 30 days after surgery.
Study variables
The primary outcome was postoperative referral to an oncologist. The study population was divided into three groups based on age (<75, 75–79, ≥80). We obtained baseline patient characteristics including gender, smoking status, alcohol consumption and body mass index (BMI), as well as patient, surgical and tumor-related characteristics including comorbidity/functional status, tumor location, surgical approach, post-operative complications, whether or not a preoperative MDT-conference was performed (according to Danish guidelines introduced in 2012 consisting of at least one colorectal surgeon, one radiologist, one oncologist and one pathologist), and the administrative geographical region of treatment. Smoking status was classified as either current smoker, former smoker, nonsmoker or unknown. Alcohol consumption was classified into four groups depending on units per week and unknown consumption. BMI was calculated from height and weight and was categorized in four groups as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–30 kg/m2) and obese (>30 kg/m2). Comorbidity was assessed using the Charlson comorbidity index (CCI) [Citation13]. CCI was calculated without contribution to the score of current cancer or age, to allow comparison of the age groups and was categorized into four groups: 0, 1, 2 and ≥3 [Citation14–16]. Functional capacity was assessed using the WHO Performance Status (PS) [Citation17]. Both PS and CCI were registered in the DCCG-database at the time of diagnosis. Tumor localization was either colon or rectum. Compromised resection was defined as having a limited surgical resection planned before surgery and not according to the general guidelines for the extent of bowel resection. Surgery could be either laparoscopic or open, the latter including laparoscopic procedures converted to open. Post-operative complications within 30 postoperative days were classified using the Clavien-Dindo classification [Citation18].
Statistics
Data were categorical and compared by Chi-square test. Possible covariates included in the models were determined by directed acyclic graphs. Univariate analysis of the possible covariates was conducted to determine significance. All significant variables were then used in a multivariate logistic regression to adjust for potential confounders. Patients with higher PS have very low tolerability of chemotherapy and are generally not offered chemotherapy, and, therefore, we conducted a subgroup analysis excluding patients with the highest PS (PS 3–4). Only cases with complete data on the primary outcome, age at the time of diagnosis, 30-days mortality, and complications, were used in the analysis. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). All reporting was in compliance with the STROBE guidelines [Citation19].
Results
A total of 15,300 patients were diagnosed with adenocarcinoma in colon or rectum from 2014 through 2016. After exclusion, 2791 patients with stage III cancer were included in the study (Supplementary material Figure S1). Patient and treatment characteristics are summarized in . There were missing data regarding 10 patients in the main category of interest, referral to an oncologist. In the univariate analyses of the potential covariates sex, smoking status, alcohol consumption, BMI, geographical region of treatment, CCI, PS, postoperative complications, compromised resection and open surgery was all significantly associated with not being referred to an oncologist and included in the multivariate logistic regression analysis. Presenting the patient case at an MDT-conference and type of cancer was found not significant.
Table 1. Patient and treatment characteristics.
Multivariate analysis
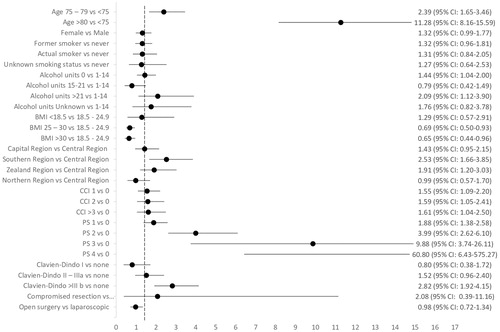
In the multivariate logistic regression, after adjustment for the covariates found statistically significant in the univariate analysis, an OR of 11.28 (95% CI: 8.16–15.59) for patients aged ≥80 years, and an OR of 2.39 (95% CI: 1.65–3.46) for patients aged 75–79 years, to not receive a referral to an oncologist compared to patients aged <75 was found (). Other factors that were associated independently with not being referred to an oncologist were: having a PS ≥1, CCI ≥1, alcohol consumption, and a having a Clavien-Dindo ≥ IIIb complication. Being treated in two of the five geographical regions was also found to be associated with no referral. Having a BMI > 25 was associated with a decreased risk of no referral. The multivariate logistic regression performed excluding patients with PS 3–4 did not change the primary outcome (data not shown). The reasons for not being referred to an oncologist were registered in the DCCG database for only nine percent of the patients and therefore did not allow statistical analysis.
Discussion
The main finding is that higher age is an individual risk factor for not being referred to an oncologist for evaluation in patients with UICC stage III colorectal cancer, despite having curative intended surgery. These results remained significant even after adjustment for comorbidities and functional status. Almost half of all patients above 80 years of age were not referred to an oncologist. This potential substandard treatment is noteworthy, especially considered, that all patients included in this study were estimated physically fit to undergo anesthesia and major abdominal surgery, although we do not have data whether the surgery was planned due to potential bowel obstruction or other tumor related symptoms. The results are consistent with other older studies evaluating the referral and receipt of adjuvant chemotherapy in patients with colorectal cancer [Citation7,Citation20–22]. Even though the elderly patients are increasingly healthier, the surgical treatment is less traumatic, and the risk of complications has been reduced, it seems that elderly patients still are not referred for guideline-recommended therapy.
Neither high-risk CRC stage II patients nor patients undergoing emergency surgery were included in the study. This decision was done to ensure methodological rigor because both of these populations requires specific considerations. The benefit of adjuvant of elderly patients with high-risk stage II disease is not clear [Citation23,Citation24] and patients who undergo emergency surgery more often have open surgery, complications, higher stage of disease and cannot follow the usual preoperative evaluation [Citation25–28].
The primary strength of the study is the comprehensive data available on each patient through a prospectively collected nationwide database with completeness above 99% [Citation12]. Further, the treatment guidelines for referral to oncological assessment remained unchanged throughout the study period, and the guidelines in Denmark for this period do not differ from international guidelines. The Danish health care system is free and with equal access to health care for all citizens, and we were able to adjust for geographical differences. Studies have shown that even in populations with equal access to health care, patients with lower income or lower socio-economic status are less likely to be referred to oncological treatment [Citation21,Citation29]. This might partly explain some of the geographical differences of referral found in the current study.
There are several limitations to the study which should be considered in interpreting the data. First of all, a reason for no referral was only available from nine percent of the patients, and therefore the reasoning behind the decisions remains unclear. Only very few studies on reasons for refusal of oncological treatment by the doctor are available. A study conducted in Canada found that 27% of patients referred to an oncologist did not receive chemotherapy [Citation30]. Eighteen percent due to the doctor’s recommendations, and the most common reason being comorbidity or a combination of comorbidity, age and frailty. Nine percent of the patients refused to receive chemotherapy following the specialist consultation. The willingness of undergoing adjuvant chemotherapy may decrease with age and may explain some of the effects. Second, data on whether a postoperative MDT conference was held were not available from the database. A preoperative MDT conference was mandatory by national guidelines during the study period; however, postoperative MDT was only recommended. Thus, we do not know the influence that a postoperative MDT conference would have on referral. This is a limitation and some of the effects may be due to unmeasured confounding. Third, data on survival were not included in this study, and therefore we do not know whether referral to an oncologist would increase the cancer-free survival compared to the patients in our study population who were not referred. Patients referred to individual specialist evaluation by an oncologist do not and should not all receive adjuvant chemotherapy, but referral of the patients is the first and crucial step to be evaluated for adjuvant chemotherapy.
According to the Danish and international guidelines for colorectal cancer within the study period, there was no definite upper age limit regarding the use of adjuvant chemotherapy, but the treatment is recommended to be individualized taking into account the overall fitness and age of each patient. The group of older patients with colorectal cancer is heterogeneous with some being healthy and independent; while others are frail, have social problems, and serious comorbidity. This is not necessarily well reflected by the chronological age, and the proportion of healthy elderly with high functional capacity is increasing. Thus, proper evaluation by an oncologist and a geriatrician after surgery is important to identify the patients which could benefit from adjuvant therapy and those it would harm.
In conclusion, this prospective nationwide cohort study on patients with UICC stage III colorectal cancer found that higher age was an individual risk factor for not being referred to an oncologist to evaluation for guideline-recommended adjuvant chemotherapy. Whether or not there is a difference in recurrence rate and survival in our study between patients referred or not needs further investigation. Further studies are needed to identify parameters that can safely select older patients who are candidates for chemotherapy regardless of age.
Author contributions
RDB: conception and study design, analysis and interpretation of data, manuscript writing, and approval of the final manuscript. MF: conception and study design, analysis and interpretation of data, manuscript writing, and approval of the final manuscript. IG: conception and study design, analysis, and interpretation of data, revising the manuscript for important intellectual content and approval of the final manuscript.
Supplemental Material
Download MS Word (43.1 KB)Acknowledgments
The authors thank the DCCG.dk for kindly providing the data. The authors do not have permission to share data, but aggregated anonymized data can be provided upon request to the author group.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sanoff HK, Carpenter WR, Stürmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30(21):2624–2634.
- Bergquist JR, Thiels CA, Spindler BA, et al. Benefit of postresection adjuvant chemotherapy for stage III colon cancer in octogenarians. Dis Colon Rectum. 2016;59(12):1142–1149.
- Hung A, Mullins CD. Relative effectiveness and safety of chemotherapy in elderly and nonelderly patients with stage III colon cancer: a systematic review. Oncologist. 2013;18(1):54–63.
- Papamichael D, Audisio R, Horiot J-C, et al. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol. 2009;20(1):5–16.
- Lund CM, Nielsen D, Dehlendorff C, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colorectal cancer: the ACCORE study. ESMO Open. 2016;1(5):e000087.
- Kahn KL. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303(11):1037.
- Serra-Rexach JA, Jimenez AB, García-Alhambra MA, et al. Differences in the therapeutic approach to colorectal cancer in young and elderly patients. Oncologist. 2012;17(10):1277–1285.
- Christensen K, Thinggaard M, Oksuzyan A, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet. 2013;382(9903):1507–1513.
- Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56.
- Iversen LH, Green A, Ingeholm P, et al. Improved survival of colorectal cancer in Denmark during 2001–2012 – the efforts of several national initiatives. Acta Oncol. 2016;55(Suppl. 2):10–23.
- Weerink LBM, Gant CM, van Leeuwen BL, et al. Long-term survival in octogenarians after surgical treatment for colorectal cancer: prevention of postoperative complications is key. Ann Surg Oncol. 2018;25(13):3874–3882.
- Ingeholm P, Gögenür I, Iversen L. Danish Colorectal Cancer Group Database. Clin Epidemiol. 2016;8:465–468.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Ostenfeld EB, Nørgaard M, Thomsen RW, et al. Comorbidity and survival of Danish patients with colon and rectal cancer from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013;5(Suppl. 1):65–74.
- Iversen LH, Nørgaard M, Jacobsen J, et al. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006—a population-based cohort study. Dis Colon Rectum. 2009;52(1):71–78.
- Baretti M, Rimassa L, Personeni N, et al. Effect of comorbidities in stage II/III colorectal cancer patients treated with surgery and neoadjuvant/adjuvant chemotherapy: a single-center, observational study. Clin Colorectal Cancer. 2018;17(3):e489–e498.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
- Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457.
- Bojer AS, Roikjær O. Elderly patients with colorectal cancer are oncologically undertreated. Eur J Surg Oncol. 2015;41(3):421–425.
- Winget M, Hossain S, Yasui Y, et al. Characteristics of patients with stage III colon adenocarcinoma who fail to receive guideline-recommended treatment. Cancer. 2010;116(20):4849–4856.
- Luo R, Giordano SH, Freeman JL, et al. Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. Oncologist. 2006;11(9):1025–1033.
- QUASAR Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029.
- Nitsche U, Stöss C, Friess H. Effect of adjuvant chemotherapy on elderly colorectal cancer patients: lack of evidence. Gastrointest Tumors. 2017;4(1–2):11–19.
- Lavanchy JL, Vaisnora L, Haltmeier T, et al. Oncologic long-term outcomes of emergency versus elective resection for colorectal cancer. Int J Colorectal Dis. 2019;34(12):2091–2099.
- Wanis KN, Ott M, Van Koughnett JAM, et al. Long-term oncological outcomes following emergency resection of colon cancer. Int J Colorectal Dis. 2018;33(11):1525–1532.
- Osagiede O, Spaulding AC, Cochuyt JJ, et al. Factors associated with minimally invasive surgery for colorectal cancer in emergency settings. J Surg Res. 2019;243:75–82.
- Degett TH, Dalton SO, Christensen J, et al. Mortality after emergency treatment of colorectal cancer and associated risk factors—a nationwide cohort study. Int J Colorectal Dis. 2019;34(1):85–95.
- Lemmens V, van Halteren AH, Janssen-Heijnen MLG, et al. Adjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands is affected by socioeconomic status, gender, and comorbidity. Ann Oncol. 2005;16(5):767–772.
- El Shayeb M, Scarfe A, Yasui Y, et al. Reasons physicians do not recommend and patients refuse adjuvant chemotherapy for stage III colon cancer: a population based chart review. BMC Res Notes. 2012;5:269.