Abstract
Objective(s)
Neoadjuvant chemotherapy (NAC) is a standard of care for locally advanced breast cancers. Adjuvant radiotherapy (RT) after NAC is an area of active research. We hypothesize overall survival (OS) is not altered by omitting RT in women with a pathologic complete response (pCR) to NAC after breast conserving survery (BCS).
Methods
Patients from the National Cancer Database who underwent NAC, BCS, and had a pCR were included. Inflammatory disease, <6 months follow up, and unknown variables were excluded. Descriptive statistics characterized the retained cohort. Logistic regression analyzed the influence of variables on the rate of RT omission. Cox proportional hazard modeling analyzed the influence of prognostic variables on OS.
Results
Of 5383 women included, 364 (7%) omitted RT. 5-year OS was 94.1% with RT, 93% without. RT omission was most likely in women >70yo (adjusted OR2.4, 95%CI 1.58–3.65, p < .0001;reference 40–49 yo), Hispanic (AOR 1.73, 95%CI 1.19–2.52, p = .0044; reference non-Hispanic), ≥20 miles from treatment facility (20–49 miles; AOR 1.45, 95%CI 1.09–1.93, p = .0109: >50 miles; AOR 2.02, 95%CI 1.42–2.87, p < .0001;reference 0–19 miles), grade 1 (AOR 4.29, 95%CI 2.16–8.51, p < .0001; reference grade 3), and clinical T4 disease (AOR 3.17, 95%CI 1.74–5.79, p = .0002; reference T0/1). Women ≥60yo (60–69: AHR 2.33, 95%CI 1.41–3.83, p = .0009:70+:AHR 2.4, 95%CI 1.24–4.62, p = .0092; reference 40–49) and with N1 and N3 disease (N1: AHR 1.67, 95% CI 2.28–3.24, p = .0034; N3: AHR3.37,95%CI2.01–5.65,p < .0001) showed increased death. Triple-positive (AHR 0.18, 95%CI 0.07–0.43, p = .0002) and HER2+ patients (AHR 0.44, 95%CI 0.30–0.64, p < .0001) had improved OS compared to triple-negative disease. No survival difference was seen with omission of RT (log-rank test: p = .1783; Cox model AHR 1.33, 95%CI 0.76–2.31, p = .3181).
Conclusion
Women ≥70, of Hispanic origin, living ≥20 miles from treatment facility, and grade 1 disease were more likely to omit RT. HER2+ patients had favorable OS, while older age and N3 disease were negative prognostic factors. Omitting RT after a pCR to NAC and BCS was not found to affect OS.
Introduction
Neoadjuvant chemotherapy (NACT) in breast cancer is increasing in frequency [Citation1] and is considered a standard of care for patients with locally advanced and inoperable tumors. Well established benefits of neoadjuvant chemotherapy include increased rates of breast conservation [Citation2–6], converting inoperable tumors to operable [Citation7], potential clearing of primary and/or axillary disease [Citation8–10], and providing prognostic information based on tumor response [Citation11].
Adjuvant radiotherapy (RT) has been proven to be effective in providing superior locoregional control as well as improved overall survival (OS) in a patient population receiving surgery as their first intervention. Specifically, for patients who underwent breast conservation surgery (BCS), the landmark Early Breast Cancer Trialists’ Collaborative Group meta-analysis showed improved local control, locoregional control, and breast cancer death when adjuvant whole breast radiotherapy was delivered versus observation in both node negative and node positive patients [Citation12]. More recent studies by Poortmas et al. and Whelan et al. both validated adjuvant breast/chest wall irradiation with elective nodal coverage in node positive and other select high risk patients, with the later showing a benefit in distant metastasis free survival [Citation13,Citation14].
Given the emergence of NACT and the compelling evidence that pathologic response correlates with disease free survival in these patients, the extent of utilization and the efficacy of adjuvant RT has been called into question. To this point, adjuvant RT recommendations after NACT have been based on clinical stage with pathologic response only recently being considered into treatment algorithms. Contemporary studies such as NSABP B-51 and Alliance A011202 are investigating the role of adjuvant RT in pathologically node negative and pathologically node positive patients after neoadjuvant chemotherapy, respectively.
To our knowledge, there is no data concerning omission of RT in patients with a pathologic complete response (pCR) after NACT and breast conserving surgery. We analyzed a cohort of patients using the National Cancer Database (NCDB) to add to the body of knowledge in this clinical scenario, and to determine whether omission of RT affects OS.
Materials and methods
The NCDB is a nationwide, facility-based joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society that reports hospital level data, representing approximately 70% of patients with newly diagnosed malignancies in the United State [Citation15]. The study was deemed exempt by our institutional review board.
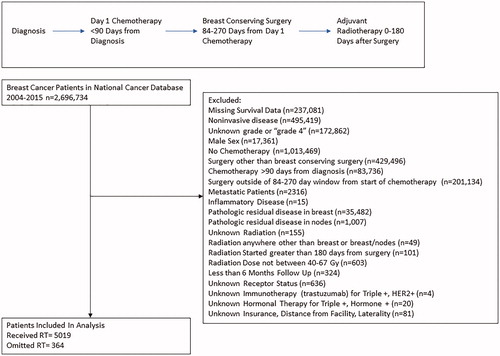
The NCDB was quired for all patients diagnosed with breast cancer between 2004–2015. Inflammatory disease, unknown stage, unknown receptor status, unknown treatment regimen, noninvasive histology, and unknown demographic variables were excluded from analysis. Women with less than 6 months follow up after surgery were excluded to limit immortal time bias [Citation16]. Included women were cT0-T4c, cN0-N3, Mx or M0 who underwent NACT and BCS, with pathology showing a pCR in both the breast and lymph nodes. NACT was defined as chemotherapy before surgery or RT and must have been started within 90 days of diagnosis to control for treatment delays and exclude any patients receiving systemic therapy for metastatic disease [Citation1,Citation17]. Surgery was limited to BCS and had to occur within 84–270 days from the start of chemotherapy to control for delays in treatment and ensure adequate NACT [Citation1]. If adjuvant RT was received, it was limited to the breast and/or lymph nodes and between 0 and 180 days from surgery. Radiation doses included boost doses if delivered, such that total doses of 40–67 Gy were included per NCCN guidelines acceptable doses [Citation18] ().
Descriptive statistics were performed to characterize the retained cohort. Differences in demographics were assessed using chi-squared test, and marginal effects on OS were tested using the log-rank test. The effect of omitting RT on OS was considered marginally using Kaplan–Meier methods. Factors that were approaching significance for survival in a univariate model were analyzed in a multivariate setting. Multivariate modeling with the Cox proportional hazard model was used to control for confounding due to other demographic and prognostic variables associated with OS. A sensitivity analysis was performed using propensity score weighting to control for potential treatment selection bias. Logistic regression was done to analyze the influence of variables on the rate of RT omission. All analysis was performed using the R statistical software, version 3.6.2 [Citation19].
For the reminder of this manuscript, immunotherapy and HER2-directed therapies are used interchangeably as HER2 directed therapies are coded as immunotherapies by the NCDB.
This study was designed in accordance with STROBE guidelines (Supplementary material) [Citation20].
Results
The study cohort included 5383 women. 364 omitted radiation. Most patients were between the ages of 40 to 69 years old (92%), white (74%), non-Hispanic (93%), and with a Charleson/Deyo comorbidity score of 0 (88%). Most women lived 0 to 19 miles from treatment facilities (77%) and had grade 3 disease (79%). Clinical T2 disease was most common (65%). Clinical N0 stage was the highest percentage at 54%, followed by N1 stage at 38%. HER2 receptor positive was the most common phenotype constituting 49% (triple positive women were 15%, other HER2+ women represented 34%). 38% of the women were triple negative, and 13% were Hormone+/HER2- (). Of women who were hormone receptor positive, 86% received hormone therapy. 73% of women with HER2+ disease received immunotherapy (i.e. trastuzumab).
Table 1. Baseline patient, tumor and treatment details.
The 5-year OS was 94% in the full cohort (95% CI: 93.0–95.1%; ). Median Follow up was 27.9 Months from landmark cutoff (Interquartile range 16.9–44.7 months). Survival of patients who received RT was 94.1% (95% CI 93.0–95.2%) and was 93% (95% CI 88.9–97.3%) for people who did not receive RT (log-rank test: p = .1783; ). A sensitivity analysis was performed using propensity score weights for RT omission. After reweighting, 5-year OS was 93.6% with RT (95% CI 89.3–98.2%) compared to 93% without RT (95% CI 88.9–97.3%). Consistent with the unweighted analysis, no effect due to omitting RT was detected (p = .7773; ). In multivariate analysis controlling for other confounders, no significant difference was detected due to omitting RT (adjusted hazard ratio 1.33, 95% CI 0.76–2.31, p = .3181; ).
Figure 2. (A) Kaplan–Meier curve for overall survival. (B) Kaplan–Meier curve for overall survival by RT. (C) Kaplan–Meier curve for overall survival by RT propensity score weighted.
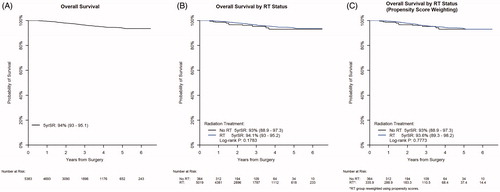
Table 2. Cox proportional hazard modeling for survival.
Age correlated with survival with women 60 years of age or older showing an increased hazard ratio for death (60 to 69, AHR 2.33, 95% CI 1.41–3.83, p = .0009; 70+, AHR 2.40, 95% CI 1.24–4.62, p = .0092) when compared to 40–49 years old as reference. Ages <40 and 50–59 did not differ from 40–49 years old. N1 and N3 disease showed an increased hazard ratio for death (N1, AHR 1.67, 95% CI 1.18–2.34, p = .0034; N3, AHR 4.48, 95% CI 2.60–7.72, p < .0001), while women with N2 (AHR 1.04, 95% CI 0.53–2.05, p = .9177) stage did not show a decreased chance of survival when compared to N0 as reference. Women with Triple positive disease (AHR 0.18, 95% CI 0.07–0.43, p = .0002) and HER2+ women (AHR 0.44, 95% CI 0.30–0.64, p < .0001) both had a significantly improved OS compared to triple negative as reference. Hormone+/HER2- women (AHR 0.82, 95% CI 0.54–1.26, p = .3641 showed no difference in OS compared to triple negative women as reference ().
Women most likely to omit radiation therapy were ones older than 70 years of age (adjusted OR 2.40, 95% CI 1.58–3.65, p < .0001; reference 40–49 yo), those with Hispanic heritage (AOR 1.73, 95% CI 1.19–2.52, p = .0044; reference non-Hispanic), those who live 32.2 kilometers (20 miles) or greater distance from treatment facility (32.2 (20 miles)-78.9 kilometers (49 miles); AOR 1.45, 95% CI 1.09–1.93, p = .0109: >78.9 kilometers (50 miles); AOR 2.02, 95% CI 1.42–2.87, p < .0001; reference 0–32.2 kilometers (19 miles)), those with grade 1 disease (AOR 4.29, 95% CI 2.16–8.51, p < .0001; reference grade 3), and those with clinical T4 tumors (AOR 3.17, 95% CI 1.74–5.79, p = .0002; reference T0/1). Patients who did not receive hormonal therapy were more likely to omit radiotherapy (AOR 7.98, 95% CI 4.93–12.93, p < .0001). Likewise, patients who did not receive immunotherapy were more likely to omit radiotherapy (AOR 1.55, 95% CI 1.13–2.13, p = .0068) ().
Table 3. Logistic regression for omission of RT.
Interaction effect testing was done to see if the effect omission of RT on OS differed due to the other confounders of age, insurance status, Charlson/Deyo comorbidity score, nodes examined, N stage, hormone receptor status, hormone therapy, and trastuzumab. No significant interactions were found for any of these factors.
Discussion
NACT has quickly become a standard of care for locally advanced breast cancer, which is illustrated by the percentage of our cohort increasing every year between 2004–2015. It is known that response to neoadjuvant chemotherapy differs based on histologic subtype, with HER2+ tumors responding at a higher rate than triple negative, which in turn respond at a higher rate than hormone positive cancers [Citation21]. High grade tumors are also known to respond more robustly than grade 1 or 2 disease [Citation22]. This cohorts’ high proportion of grade 3, HER2+ and triple negative tumors most likely reflects this tendency for increased response. Our cohort also has a large proportion of early stage tumors. Goorts et al. previously showed that clinical T stage is a significant predictor for pCR rates, with lower T stage having higher rates of pCR [Citation23]. Thus, the high proportion of early stage patients could be expected.
Women ≥ 70 years old were more likely to omit RT than younger women. Women who did not receive the expected systemic therapies (immunotherapy for HER2+/Triple positive, hormonal therapy for Hormone+/Triple +) were more likely to omit RT than those who did. Although comorbidity score was used to control for overall health of patients, it is possible that this reflects a population of patients deemed too ill after NACT and surgery to benefit from adjuvant RT. There is data for omission of adjuvant RT in woman > 70 years old, albeit in patients with vastly different biological disease [Citation24]. It is possible that this data is being extrapolated to elderly woman with an excellent response to chemotherapy. A similar thought process can be used to explain the increased likelihood of omitting RT in grade 1 disease seen in this cohort, as data for omission of adjuvant therapy is in low grade disease. However, there were only 1% (n = 65) of patients with grade 1 disease, so this effect may not be replicable in further studies. Similarly, the surprising result that patients with T4 disease are more likely to omit RT may be statistical chance, as T4 disease made up 2% (n = 107) of the cohort. Distance from treatment facility also predicted for increase omission of RT. This agrees with multiple studies showing increased distance from treatment facility is inversely associated with utilization of adjuvant breast irradiation [Citation25–28].
HER2+ patients showed a significantly improved survival in this cohort. This phenomenon could be related to the well-established idea that HER2+ disease responds exquisitely to targeted therapies (i.e. trastuzamab) and that pathologic response correlates with outcomes [Citation11]. This alone cannot explain the significantly improved survival for HER2+ women, though, as all women in this cohort experienced a complete pathologic response. It could be postulated that some proportion of patients developed metastases and that HER2+ metastatic disease has been shown to have improved OS compared to HER2- disease when targeted agents are used [Citation29]. Whether this effect is strong or prevalent enough to influence this cohort is difficult to establish. Interestingly, no survival benefit was seen in HER2+ or Triple positive women who received immunotherapy on univariate analysis when compared to those who didn’t (). One may argue that the benefit of immunotherapy in this population may be diluted as every patient has achieved a pathologic complete response regardless of the type of systemic therapy, and that is what may ultimately drive outcomes. Women ≥ 60 were more likely to die than younger women in this cohort. Traditionally, younger women were shown to have worse OS as their tumors were more likely to be high grade, hormone receptor negative, HER2+ and larger/more advanced at presentation. These previously poor prognostic factors, particularly high grade and HER2+, again show a higher response to NACT, conferring decreased risk of locoregional failure. Increased risk of death at an elevated age could reflect population with more medical comorbidities but could illustrate the changing landscape of prognostic variables in the area of NACT and targeted agents.
Adjuvant RT was not found to affect survival in this cohort. To our knowledge, this is the first study analyzing patients who underwent adjuvant RT after BCS with a PCR to NACT, but adjuvant RT has been studied for postmastectomy patients in that scenario. McGuire et al. reported improved OS and local control for locally advanced patients who had adjuvant RT after pCR and mastectomy. Stage I and stage II patients did not benefit [Citation30]. This differs from our study in multiple ways. First, we did not show any benefit to RT on interaction studies with staging variables. It is important to note that a much arger proportion of our cohort was early stage compared to McGuire et al. The patients in our cohort are biased toward earlier T stages as they all underwent BCS, while the patients in McGuire et al. underwent mastectomy. Additionally, only 3% of patients in McGuire et al. received HER2 directed therapy even though 17% of the patients were HER2+ (a 17.6% utilization rate for HER2+ patients, while in our cohort it was 73%). The traditional chemotherapies used were detailed previously, but shortly, 95% percent received an anthracycline chemotherapy and 42% received taxanes [Citation31]. As discussed earlier, it is difficult to know if increased utilization of HER2 directed therapies or more modern chemotherapy regimens would have changed their outcomes considering all of these patients achieved pCR.
This is a large, retrospective analysis of the NCDB and carries with it the inherent limitations that come with these investigations. The data lacks granularity in terms of chemotherapy agents. It only reports OS data, making local, locoregional, and distant control outcomes impossible to analyze which are significant factors to consider when it comes to radiotherapy and breast cancer. The database codes for primary treatment modalities only, so subsequent disease modifying treatments are unknown (i.e. adjuvant HER2 directed therapies, systemic therapy for metastatic disease developed after treatment). There is also somewhat limited follow up data as >50% of this cohort comes from 2014 and 2015, the last two years queried, resulting in a median follow up time of 27.9 months. Studies that have been successful in showing a survival benefit in breast cancer have needed significantly longer follow up. For instance, the EBCTCG metanalysis that showed a breast cancer mortality benefit for adjuvant radiotherapy in early stage patients had a median follow up of 9.5 years [Citation12] The sample size of 5383, of which 364 omitted radiotherapy, is modest compared to other studies which have shown differences in survival for radiotherapy in breast cancer. Also,as discussed previously, there is a large proportion of patients with early stage breast cancer in this cohort. Although T stage and tumor size were not found to be significant for survival on univariate analysis, it is reasonable to think that a larger proportion of T3 and T4 patients could allow for a subtle survival difference to be elucidated.
This analysis of the NCDB provides data suggesting that adjuvant RT in patients with pCR after NACT and BCS may not improve OS. While we would not recommend omitting RT in this population without level I evidence, this data is hypothesis generating and shows that omitting adjuvant RT in this population deserves further prospective study.
Supplemental Material
Download MS Word (30 KB)Disclosure statement
The authors report no conflicts of interest.
References
- Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015;121(15):2544–2552.
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Cin Oncol. 1997;15(7):2483–2493.
- Golshan M, Cirrincione CT, Sikov WM, Alliance for Clinical Trials in Oncology, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat. 2016;160(2):297–304.
- Barranger E, Antomarchi J, Chamorey E, et al. Effect of neoadjuvant chemotherapy on the surgical treatment of patients with locally advanced breast cancer requiring initial mastectomy. Clin Breast Cancer. 2015;15(5):e231–e235.
- Hage AN, Capriccioso C, Brennan J, et al. Impact of neoadjuvant chemotherapy on surgical outcomes among patients with hormone receptor positive breast cancer. J Surg Oncol. 2017;116(6):665–670.
- Asselain B, Barlow W, Bartlett J, et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39.
- Wang M, Hou L, Chen M, et al. Neoadjuvant chemotherapy creates surgery opportunities for inoperable locally advanced breast cancer. Sci Rep. 2017;7:44673.
- Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23(12):2694–2702.
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384.
- Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13(1):228–233.
- Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30(32):3960–3966.
- Early Breast Cancer Trialists' Collaborative Group; Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716.
- Poortmans PM, Collette S, Kirkove C, EORTC Radiation Oncology and Breast Cancer Groups, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–327.
- Whelan TJ, Olivotto IA, Parulekar WR, MA.20 Study Investigators, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307–316.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722–1728.
- Park HS, Gross CP, Makarov DV, et al. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(5):1365–1373.
- Sanford RA, Lei X, Giordano SH, et al. Impact of delayed neoadjuvant systemic chemotherapy on survival outcomes in breast cancer patients. J Cin Oncol. 2016;34(15_suppl):1038–1038.
- Network NCC. Breast Cancer (Version 2.2020). [cited 2020 Feb 28] Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf.
- R Core Team. R: A language and environment for statistical computing. R foundation for Statistical Computing. 2018. http://finzi.psych.upenn.edu/R/library/dplR/doc/intro-dplR.pdf
- von Elm E, Altman DG, Egger M, STROBE Initiative, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Swisher SK, Vila J, Tucker SL, et al. Locoregional control according to breast cancer subtype and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast-conserving therapy. Ann Surg Oncol. 2016;23(3):749–756.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172.
- Goorts B, van Nijnatten TJA, de Munck L, et al. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2017;163(1):83–91.
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387.
- Dragun AE, Huang B, Tucker TC, et al. Disparities in the application of adjuvant radiotherapy after breast-conserving surgery for early stage breast cancer: impact on overall survival. Cancer. 2011;117(12):2590–2598.
- Schroen AT, Brenin DR, Kelly MD, et al. Impact of patient distance to radiation therapy on mastectomy use in early-stage breast cancer patients. J Cin Oncol. 2005;23(28):7074–7080.
- Celaya MO, Rees JR, Gibson JJ, et al. Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominantly rural population (United States). Cancer Causes Control. 2006;17(6):851–856.
- Voti L, Richardson LC, Reis IM, et al. Treatment of local breast carcinoma in Florida: the role of the distance to radiation therapy facilities. Cancer. 2006;106(1):201–207.
- Dawood S, Broglio K, Buzdar A, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Cin Oncol. 2010;28(1):92–98.
- McGuire SE, Gonzalez-Angulo AM, Huang EH, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68(4):1004–1009.
- Gonzalez-Angulo AM, McGuire SE, Buchholz TA, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Cin Oncol. 2005;23(28):7098–7104.