Abstract
Background
Pediatric melanoma may have a different biological background and more favorable prognosis compared with melanoma in adults. The aim of this study was to investigate melanoma in children and adolescents in the Finnish population in terms of incidence, clinical course, treatment, prognosis and BRAFV600E-, ALK- and PD-L1-positivity of the primary tumors.
Materials and Methods
Primary tumor samples and clinical records of all patients aged 0-19 years diagnosed with cutaneous melanoma in Finland in 1990–2014 were collected using the Finnish Cancer Registry database, Finnish hospitals and private pathology laboratories. BRAFV600E, ALK and PD-L1 were analyzed from 54 primary tumors and BRAFV600E from six metastasis samples.
Results
A total of 122 patients diagnosed with cutaneous melanoma were retrieved from the Cancer Registry database. The primary tumor samples of 73 patients were obtained for the review, and 56 cases were included in the study. The incidence of pediatric melanoma increased from 0.02 to 0.1/100 000 during the period 1990–2014. Spitzoid melanoma was the most common subtype (66%). The 10-year cancer-specific survival (CSS) was 88.7% in all patients. The 10-year-CSS did not differ in SLNB-positive or -negative groups. BRAFV600E was positive in 48%, ALK in 9% and PD-L1 in 2% of the tumors. BRAFV600E mutation was associated with 83% of melanoma deaths.
Conclusions
Young melanoma patients had more favorable prognosis and a different staining profile for BRAFV600E, ALK, and PD-L1 in primary tumor than reported in adults. SLNB status was not an indicator for survival. BRAFV600E-positive patients have worse prognosis and could benefit from surveillance and treatment similarly to adults.
Introduction
Pediatric melanoma is a rare malignancy, and therefore its diagnosis and treatment is challenging for pathologists and clinicians [Citation1,Citation2]. Despite increasing numbers of publications concerning pediatric melanoma, no specific guidelines for treatment have been established, and the children are treated similarly with adults [Citation2,Citation3]. However, it is being debated whether highly invasive treatments, such as complete lymph node dissection (CLND) following a positive sentinel lymph node biopsy (SLNB) should be performed for young melanoma patients in all cases, especially with Spitzoid melanomas [Citation2,Citation3].
In our previous study, we observed that melanomas of children under 16 years of age were more often amelanotic and had higher Breslow thickness compared with the Breslow thicknesses reported in adults [Citation4]. The young melanoma patients also had a higher rate of positive SLNBs in biopsies, despite having a good prognosis [Citation4].
The survival of patients with metastatic melanoma has improved significantly after approval of new targeted and immunotherapies [Citation5–8]. The discovery of BRAF mutations in approximately 50% of the primary tumors of the melanoma patients enabled the development of BRAF/MEK inhibitors, which greatly increased the survival of the patients harboring BRAFV600 mutations [Citation7,Citation9]. Also, introduction of immunotherapy with CTLA-4 and PD-1 inhibitors further increased the survival of the melanoma patients [Citation5,Citation6,Citation8]. Anaplastic lymphoma kinase (ALK) is a tyrosine kinase, and when mutated and genetically fused, promotes cell division and survival through Ras/ERK and JAK/STAT pathways [Citation10]. ALK kinase fusions are found in Spitzoid tumors [Citation11].
To investigate, whether pediatric melanomas harbor these therapeutic targets and if they serve as biomarkers for the outcome, we analyzed the primary tumor samples of Finnish pediatric and adolescent cutaneous melanoma patients immunohistochemically for BRAFV600E, PD-L1 and ALK. We combined the data from the immunohistochemical (IHC) stainings with the clinical data of the patients to review the given treatments and outcomes. With this study, we aimed for understanding the behavior of different melanoma subtypes in children to facilitate the clinical decision making while aiming for more personalized treatments.
Material and methods
Patient selection
All patients 0–19 years of age diagnosed with cutaneous malignant melanoma during the years 1990–2014 were retrieved from the Finnish Cancer Registry database. Primary tumor and metastasis samples and histopathological reports were collected from the institutions where the patients had originally been diagnosed or treated using the information from the Finnish Cancer Registry database. These institutions included hospitals, biobanks and private laboratories in Finland.
Our study was approved by the Ethics Committee of the Hospital District of Southwest Finland, and by the National Institute for Health and Welfare including a comment by a Data Protection Officer (Licenses ETMK:140/1803/2014 and THL/552/5.05.00/2016). The primary tumor samples were collected with the permission of Valvira National Supervisory Authority for Welfare and Health (License 6479/06.01.03.01/2016). Research permit for the use of Helsinki University Hospital archives of clinical data was also obtained (HUS/83/2019).
Clinicopathological analysis of tissue samples
The hematoxylin and eosin (HE) stained tumor tissue samples were independently reviewed by two dermatopathologists (LT and SJ). For the tissue samples where only formalin-fixed paraffin embedded (FFPE) tissue blocks were available, new sections were made and stained with HE.
IHC stainings for BRAFV600E (Roche, Cat# 790-5095, RRID: AB_2833072) and ALK (Roche, Cat# 790-4796, RRID: AB_2833073) were performed with BenchMark ULTRA IHC/ISH system using OptiView DAB IHC Detection Kit (Roche, Cat# 760-700, RRID: AB_2833075). PD-L1 stainings (Agilent, Cat# GE00621-2, RRID: AB_2833074) were made using the appliances and detection kit mentioned above, and 1% threshold was used for positivity. Positive control slides were made according to the manufacturer’s protocol.
The corresponding clinical data for melanoma patients was obtained from the University hospitals where the patients had been treated. Tumor staging was performed using American Joint Committee on Cancer (AJCC) 8th Edition staging at the time of diagnosis [Citation13]. Melanoma subtypes were classified by dermatopathologists using WHO Skin Tumors 4th Edition criteria [Citation14].
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics for Windows V26 (https://www.ibm.com/products/spss-statistics?mhsrc=ibmsearch_p&mhq=spss, RRID: SCR_002865). Kaplan–Meier estimates for cancer-specific survival (CSS) were calculated by time between diagnosis and death, and melanoma-specific disease-free survival (DFS) were calculated from the time of diagnosis to the time of the first melanoma metastasis or recurrence. If no melanoma-related death or metastasis occurred during the follow-up time, the patient was censored. Associations between age group, stage and BRAFV600E-positivity with survival were analyzed using log-rank test. Mann–Whitney U test, chi-square test and Kruskall–Wallis test were also used. P-values less than 0.05 were considered statistically significant.
Results
Characteristics of study cohort
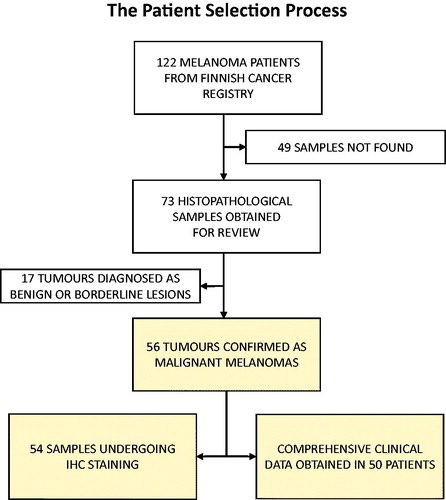
In total 122 patients aged 0–19 years diagnosed with malignant melanoma were registered in the Finnish Cancer Registry during the years 1990–2014. Six of these patients had died of metastatic melanoma, and one patient had died of a glioblastoma 15 years after the diagnosis of Spitzoid melanoma. A flowchart presenting the patient selection procedure is displayed in .
After a nationwide search, 73 primary melanoma samples of these 122 patients were obtained for a review. After independent analysis of two dermatopathologists, 56 cases out of 73 were considered as malignant melanomas and were included into the study (). The tumors which were not included in the study were diagnosed either benign nevi or melanocytic tumors of uncertain malignant potential (MELTUMPs) by the dermatopathologists. There were no melanomas which had developed in congenital nevi, and no family history of melanoma was reported.
Table 1. Patient and primary melanoma characteristics at the time of diagnosis.
Clinical data of 50 patients were retrieved from the hospitals databases. However, histopathological diagnosis reports and HE-slides were obtained from all the 56 patients. In 54 cases, primary tumor material was obtained for IHC analyses. Survival analyses were performed with stage I-IV patients (n = 53), and three in situ melanomas were not included in the survival analyses ().
The incidence of pediatric melanoma increased by time in the cohort of 56 patients. Only four cases of melanoma were diagnosed in 1990–1994 compared to 26 cases in 2010-2014. This corresponds to the incidence rate of 0.02 vs. 0.1 per 100 000, indicating a five-fold increase in the incidence rates between 1990 and 1994 and 2010–2014. Similar trend was seen also in Finnish Cancer Registry data of all 122 patients (Figure S1).
The distribution of melanomas between both male and female was 48.2% and 51.8%, respectively. Seven patients (12.5%) were under 11 years old, and five of them were boys (71.4%). The mean age at diagnosis was 15.5 years. Patient and primary tumor characteristics at the time of diagnosis are shown in .
Disease-free and cancer-specific survival
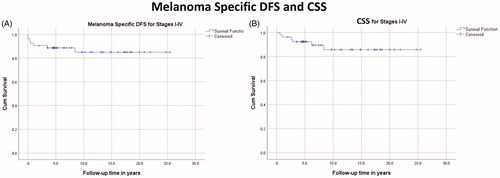
Melanoma specific 1-year-DFS and CSS were 90.6% and 96.2%, respectively. 5-year-DFS and -OS were 88.6% and 92.5%, respectively. 10-year-DFS and CSS were 86.8% and 88.7%, respectively. No deaths occurred after 10 years. The Kaplan–Meyer estimates for both DFS and CSS are shown in .
Cancer-specific survival by stage
In children and adolescents, the most common melanoma AJCC stages were I and III covering 46.4% and 26.8% of the population, respectively. Distribution and survival of different stage groups are demonstrated in .
Figure 3. (A) Distribution of melanoma stages (n = 56). (B) Kaplan–Meier estimate for melanoma-specific CSS in different stages (n = 53). In situ (Tis) melanomas (n = 3) were not included in the survival analysis.
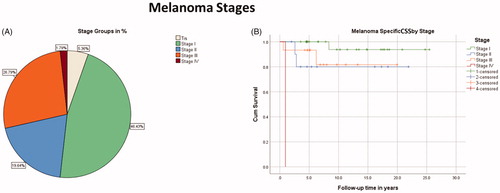
In contrast to the excellent 10-year-CSS of the patients under 11 years old (100%), they presented with higher disease stages than the older patients in the 16-19 years age group, whose 10-year-CSS was 92.9%. The mean age of the patients with stage III disease was significantly lower compared with stage I patients, 12 vs. 17 years, respectively (p = 0.004, adjusted, Bonferroni corrected, n = 53). The prognosis was worse in the age group of 11-15 years, whose 10-year-CSS was 77.8%. Kaplan–Meier estimates and distribution of stage groups by age are shown in Figure S2.
Surgical treatment and staging
In 54 patients, surgical treatment of primary melanoma was excision with at least 1-2 cm margins. Two patients presented with either distant metastasis or clinically detectable lymph node metastasis at the time of diagnosis, and therefore their primary tumors and metastases were excised only for diagnostic purposes. SLNB was introduced in Finland from 2000-2002 onwards. Twenty-seven patients (49%) underwent SLNB and metastatic sentinel nodes were detected in 12 of them (44%). All SLNB-positive patients underwent CLND and additional non-sentinel metastases were detected in two of them (17%). Before the era of SLNB, four patients (7%) underwent elective lymph node dissection (ELND) and regional lymph node metastasis was detected in one of them. One patient (2%) with metastatic lymph nodes underwent therapeutic lymph node dissection (TLND). For one patient, no information on whether SLNB or evacuation was performed was found (Figure S3).
There was no difference in the DFS or CSS among patients with positive or negative SLNB during the follow-up, both groups demonstrating 100% melanoma-specific DFS and CSS rates (Figure S3). The Breslow thickness was higher for the melanomas with positive SLNB compared to negative SLNB result, the mean being 4.8 mm vs. 1.6 mm, respectively (p = 0.00017, 2-tailed, n = 27). SLNB positive patients were also younger than SLNB-negative patients (p = 0.011, 2-tailed, n = 27). The mean age for SLNB-positive patients was 12 years vs. 16 years for SLNB-negative patients.
Medical oncological therapies
Medical oncological therapies were given to 16 patients and 11 of them had positive SLNB. One patient did not have positive SLNB, but she had a Clark level 4, Breslow 4,6mm Spitzoid melanoma without ulceration and mitotic rate of 2/mm2. These 12 patients received adjuvant Interferon-α (IFN-α) treatment apart from two patients, who received combination adjuvant therapy. The other received adjuvant combination therapy of IFN-α and Dacarbazine (DTIC), and the other was treated with IFN-α and the combination of dacarbazine, vincristine, lomustine, and bleomycin (BOLD). Adjuvant radiation therapy along with IFN-α adjuvant therapy was given to one patient with Clark level 4, Breslow 1,6mm Spitzoid melanoma without ulceration, mitotic rate of 3/mm2 and total nodal status of 6/14. Distribution of oncologic therapies in this patient population is shown in Figure S4.
Four patients received combination of chemo- and immunotherapies for their metastatic melanoma, as well as adjuvant or palliative radiotherapy. Therapies received by these patients are shown in Table S1. Only two patients in our study were treated with BRAF/MEK inhibitors (vemurafenib, dabrafenib). The other had also <1% PD-L1-positivity in the primary tumor cells and received additional therapy with CTLA-4 antibody (ipilimumab) and PD-1 inhibitor (pembrolizumab).
BRAFV600E, ALK, and PD-L1 immunohistochemistry
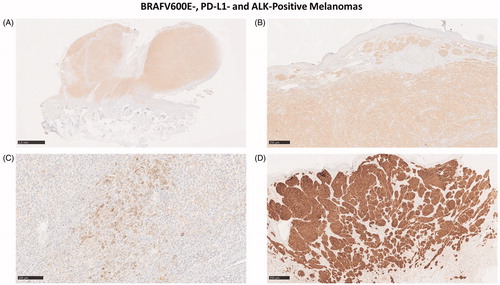
In total, 54 primary melanoma samples were analyzed with IHC for BRAFV600E, PD-L1, and ALK. Six metastases were analyzed for BRAV600E. The IHC results are shown in Table S2. Representative IHC stainings of BRAFV600E-, ALK- and PD-L1-positive melanomas are shown in .
Figure 4. (A–C) BRAFV600E-positive (A, B) nodular melanoma located behind the ear of an 18-year-old male who died of metastatic melanoma. In addition to BRAFV600E, the tumor showed a weak PD-L1-positivity in <1% of the tumor cells (C). (D) ALK-positive melanoma in the foot of a 16-year-old female demonstrates the typical wedge-shaped architecture of Spitzoid melanoma.
BRAFV600E
In total 26 melanomas (48%) stained positive for BRAFV600E (Roche, Cat# 790-5095, RRID: AB_2833072). Two patients with BRAFV600E had also ALK-positive (Roche, Cat# 790-4796, RRID: AB_2833073) melanomas. Four out of six patients who died of metastatic melanoma, and whose samples were stained with IHC, had BRAFV600E mutation in their primary tumors. One of these patients showed additional <1% PD-L1-positivity (Agilent, Cat# GE00621-2, RRID: AB_2833074) in the tumor cells. Ten-year-CSS was worse in patients with BRAFV600E-positive primary tumor and/or metastasis, 80.0% vs. 96.4% for the patients without BRAFV600E mutation (p = 0.081, 95% CI: 20.2–24.7 years, n = 52).
The mean age was 17 years (range 11-19 years) among BRAFV600E positive patients. Mean Breslow thickness for BRAFV600E-positive tumors was 2.4 mm (range 0.2-7.0 mm). There were no patients under 11 years who had BRAFV600E mutation in their primary tumor. All three in situ melanomas were BRAFV600E-positive.
ALK kinase fusions
Five melanomas (9%) showed strong ALK expression in majority of the tumor cells. None of the melanomas analyzed had areas of partial expression of ALK. The mean age of the patients with ALK was 14 years (range 9–18 years). Two patients had also BRAFV600E mutation. All ALK-positive melanomas were Spitzoid melanomas. The average Breslow thickness was 3.2 mm (range 1.2–6.0 mm).
PD-L1
Only one 18-year-old male patient (2%) who died of metastatic melanoma showed weak, <1% PD-L1-positivity in the tumor cells. The patient had also BRAFV600E mutation in his tumor. The Breslow thickness of the tumor was 7.0 mm, which was also the highest Breslow thickness among all BRAFV600E-positive melanomas. The presence of tumor-infiltrating lymphocytes (TIL’s) in the stroma were low when assessed with the scale of low-medium-high. The PD-L1-positivity seen in non-tumor cells was virtually non-existing ().
IHC-negative melanomas
A significant proportion, 25 melanomas (46%) stained negative for BRAFV600E, PD-L1 and ALK, therefore being called IHC-negative. The mean age was 15 years (range 5–19 years). All patients under 11 years old were IHC-negative. The mean Breslow thickness was 2.6 mm (range 0.2–15.3 mm). One patient died of metastatic IHC-negative melanoma, and another patient developed a cutaneous metastasis of melanoma close to the site of the primary tumor four months after surgery but did not show signs of disease progression during the rest of the eight-year follow-up time.
Metastases
Six metastasis samples from the patients who died of metastatic melanoma were stained with BRAFV600E. This was also to confirm, that our primary melanoma sample was the actual primary tumor which metastasized and eventually lead to death of the patient. BRAFV600E stained positive in all cases where the primary melanoma was BRAFV600E-positive, and in the metastasis of one patient whose primary tumor sample was not available for IHC-analysis. In total, five out of six melanoma metastases were BRAFV600E-positive. In the IHC-negative case, the metastasis was also negative for BRAFV600E.
Discussion
BRAFV600E-positivity of 48% was similar as previously reported for the melanoma in adults [Citation9]. Interestingly, in five out of six (83%) lethal cases the primary tumor and/or the metastases were BRAFV600E-positive. All these patients were over 11 years old, and therefore the likelihood for mutagenic environmental factors, such as UV exposure, is greater than for the younger patients. A significant part of the melanomas in adolescents could resemble the pathogenesis described in the literature, where a BRAF mutated pigmented lesion may develop into malignant melanoma when the mutational burden increases [Citation12,Citation13]. In addition to increased risk for metastasis, the 10-year-CSS for BRAFV660E-positive patients was worse than for the patients without BRAFV600E, being 80% vs. 96%, respectively. Therefore, the BRAFV600E-positive patients could benefit from being treated and surveyed similarly as adults. Also, analyzing the tumor samples for mutations using PCR method instead of only IHC could bring additional information on other V600 mutations, such as BRAFV600K.
When comparing our results with the literature, the melanomas in children and adolescents differ from the melanomas in the adults with respect to ALK- and PD-L1-positivity. In melanomas of adults, previous studies have reported 2-3% ALK-positivity [Citation14,Citation15]. In our study, 9% of melanomas were ALK-positive. The higher relative number of ALK-positive melanomas in young patients could be explained by Spitzoid features of the melanomas, since the level of 10% ALK-positivity has been reported in Spitzoid tumors [Citation16]. However, Spitzoid melanomas will probably harbor also other pathological genomic alterations besides ALK. Therefore, we would not suggest using ALK alone as a biomarker for improved outcome in pediatric melanoma. Since the diagnosis of the Spitzoid melanoma is still histopathological, it can be hypothesized that one subtype of Spitzoid melanomas may be caused by ALK mutations.
Only one patient in our study showed weak, less than 1% PD-L1-positivity. In adults, PD-L1-positivity varies significantly according to the thresholds used but has been reported to rise to 53% when using 1% threshold [Citation17]. Despite the possible variance, the rates observed in our study are much lower in young patients than reported in their adult counterparts. Whether this is due to the differences in the host immune responses or in the tumor biology remains unknown.
Nearly a half, 46% of our patients had BRAFV600E-, PD-L1- and ALK-negative primary melanomas. This included all patients under 11 years of age, who comprised 11% of the IHC-stained samples. No deaths occurred in this group of prepubertal patients either, which raises again questions on the biology behind these lesions of the very young melanoma patients. All melanomas of the patients under 11 years had Spitzoid features.
An interesting finding in the present study was that children and adolescents had a positive result in 44% of the SLNBs performed. This is a significantly larger proportion than reported in the adult population, in which SLNB-positivity is between 10-30% depending on the type of melanoma [Citation18]. The 10-year-CSS was 100% in both SLNB-positive and -negative groups and in this study SLBN was not an indicator of survival. The young patients also had an increased melanoma-specific 10-year-CSS of 88.7%, corresponding to the survival rate reported in the adults with negative SLNB [Citation18]. This could be due to the larger number of Spitzoid melanomas in the young, as atypical Spitzoid tumors often metastasize to the sentinel lymph node without worsening the prognosis [Citation19]. Children with Spitzoid melanomas might therefore have improved prognosis despite the lymph node involvement.
Melanoma specific 10-year-CSS was higher in stage III patients compared with stage III patients in Swedish adult population. A publication on adult melanoma patients in Finland classified with AJCC 8th criteria was not available for comparison. The 10-year-CSS in young patients was 100% in stages IIIA and IIIB, whereas in adults it was 80% and 55% correspondingly. Also, in stage IIIC the 10-year-CSS was higher in children and adolescents compared to the adults, 78% vs. 43%, respectively [Citation20]. The improved survival of this level could be explained with the differences in tumor biology, especially between Spitzoid and non-Spitzoid melanoma subtypes. In our study, 10-year-CSS was slightly worse in stage II compared to stage III, which emphasizes the need of critical assessment for the use of SLNB in the pediatric patients, especially in very young children. The oncological treatments given to the young patients should also be considered carefully.
The patients under 11 years old were determined prepubertal based on the likelihood of these young patients to not have reached Tanner stage 2 [Citation21]. We included patients up to 19 years of age, since by including a wide range of adolescents, we might observe the differences in the melanoma behavior between the age groups. Limitations of this study were the small number of patients who died of metastatic melanoma, and not having all the 122 primary tumor samples for the review. Since the cutaneous melanoma is extremely rare among the youngest patients, it was not possible to always show statistically significant results, for example between the survival rates. Obtaining the clinical data from all the 122 patients registered in the Finnish Cancer Registry could have been useful for comparing, e.g., the stages and survival to those of our study cohort. However, this might have led the interpretation of the results in false direction, since we could not have been sure that the original histopathological diagnosis was correct without reviewing the original primary tumor. Nevertheless, our nationwide study still provided us with a substantial number of patients to present relevant findings.
Conclusion
The prognosis of melanoma in children and adolescents is more favorable than reported in adults. The result of the SLNB did not affect the survival in this study. To avoid misinterpretation of melanocytic tumors of children and adolescents, the diagnosis should be made by at least two experienced dermatopathologists.
Primary melanomas in children and adolescents have a different IHC profile. Children under 11 years old had BRAFV600E-negative Spitzoid melanomas and presented with 100% 10-year-CSS. ALK was found in spitzoid melanomas, and PD-L1 was seldom expressed in the primary tumors of children and adolescents.
The tumors of the adolescents with BRAFV600E mutation had worse prognosis, and BRAFV600E mutation was associated with 83% of lethal cases. BRAFV600E-positive patients could benefit from surveillance and treatment similarly to adults.
Author contributions
Supplemental Material
Download MS Word (14 KB)Supplemental Material
Download MS Power Point (243.7 KB)Supplemental Material
Download MS Power Point (188.1 KB)Supplemental Material
Download MS Power Point (129 KB)Supplemental Material
Download MS Power Point (64.8 KB)Acknowledgements
The authors thank Laboratory Manager Sinikka Collanus and Medical Cell Biologist Minnamaija Lintunen for preparing the immunohistochemical stainings, colleagues and staff in Finnish hospitals and pathology laboratories for their contributions for collecting the data, Dr. Harry Kujari for his assistance with digital pathology and sample collection, and Dr. Tero Valhberg for his assistance with statistical analyses.
Micaela Hernberg: BMS, MSD, Novartis, Roche, Sanofi, Pierre Fabre and Varian (consultations, lectures). Pia Vihinen: Pierre-Fabre, BMS, Amgen, Sanofi and Roche (congress fees, travel expenses). Others declare no conflicts of interests.
ER, IK and VMK planned the study. ER collected and analyzed the data, performed statistical analyses and was responsible for writing the manuscript. SJ and LT analyzed the histopathological samples. MH and PV collected and analyzed the oncological data. IK and VMK supervised the study. All authors participated in writing and critical reviewing the manuscript.
Disclosure statement
Micaela Hernberg: BMS, MSD, Novartis, Roche, Sanofi, Pierre Fabre and Varian (consultations, lectures). PiaVihinen: Pierre-Fabre, BMS, Amgen, Sanofi and Roche (congress fees, travel expenses). Others declare no conflicts of interests.
Additional information
Funding
References
- Aldrink JH, Polites S, Lautz TB, et al. What's New in Pediatric Melanoma: An Update from the APSA Cancer Committee. J Pediatr Surg. 2019.DOI:10.1016/j.jpedsurg.2019.09.036
- Barnhill RL. The Spitzoid lesion: rethinking Spitz tumors, atypical variants, 'Spitzoid melanoma' and risk assessment. Mod Pathol. 2006;19(Suppl 2):S21–S33.
- Coit DG, Ernstoff MS, Busam KJ. Is pediatric melanoma always malignant?. Cancer. 2013;119(22):3910–3913.
- Rousi E, Koskivuo I, Kaarela O, et al. Clinical and Pathological Aspects of Melanoma among Children in Finland. Acta Derm Venereol. 2016;96(5):718–720.
- Mandalà M, Tondini C, Merelli B, et al. Rationale for New Checkpoint Inhibitor Combinations in Melanoma Therapy. Am J Clin Dermatol. 2017;18(5):597–611.
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551.
- Kaufman HL, Kirkwood JM, Hodi FS, et al. The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol. 2013;10(10):588–598.
- Franklin C, Livingstone E, Roesch A, et al. Immunotherapy in melanoma: Recent advances and future directions. Eur J Surg Oncol. 2017;43(3):604–611.
- Mandalà M, Voit C. Targeting BRAF in melanoma: biological and clinical challenges. Crit Rev Oncol Hematol. 2013;87(3):239–255.
- Roskoski R. Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacol Res. 2013;68(1):68–94.
- Busam KJ, Kutzner H, Cerroni L, et al. Clinical and pathologic findings of Spitz nevi and atypical Spitz tumors with ALK fusions. Am J Surg Pathol. 2014;38(7):925–933.
- Shain AH, Bastian BC. From melanocytes to melanomas. Nat Rev Cancer. 2016;16(6):345–358.
- Shain AH, Yeh I, Kovalyshyn I, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med. 2015;373(20):1926–1936.
- Busam KJ, Vilain RE, Lum T, et al. Primary and metastatic cutaneous melanomas express ALK through alternative transcriptional initiation. Am J Surg Pathol. 2016;40(6):786–795.
- Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116
- Wiesner T, Kutzner H, Cerroni L, et al. Genomic aberrations in spitzoid melanocytic tumours and their implications for diagnosis, prognosis and therapy. Pathology. 2016;48(2):113–131.
- Madore J, Vilain RE, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28(3):245–253.
- van Akkooi AC, Verhoef C, Eggermont AM. Importance of tumor load in the sentinel node in melanoma: clinical dilemmas. Nat Rev Clin Oncol. 2010;7(8):446–454.
- Lallas A, Kyrgidis A, Ferrara G, et al. Atypical Spitz tumours and sentinel lymph node biopsy: a systematic review. Lancet Oncol. 2014;15(4):e178–183–e183.
- Isaksson K, Katsarelias D, Mikiver R, et al. A population-based comparison of the AJCC 7th and AJCC 8th editions for patients diagnosed with stage III cutaneous malignant melanoma in Sweden. Ann Surg Oncol. 2019;26(9):2839–2845.
- Brix N, Ernst A, Lauridsen LLB, et al. Timing of puberty in boys and girls: a population-based study. Paediatr Perinat Epidemiol. 2019;33(1):70–78.